Armodafinil, Sunosi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modafinil/Armodafinil (Provigil ® /Nuvigil
Drug and Biologic Coverage Policy Effective Date ............................................ 7/1/2020 Next Review Date… ..................................... 7/1/2021 Coverage Policy Number .................................. 1501 Modafinil / Armodafinil for Individual and Family Plans Table of Contents Related Coverage Resources Coverage Policy ................................................... 1 Obstructive Sleep Apnea Treatment Services FDA Approved Indications ................................... 2 Recommended Dosing ........................................ 3 General Background ............................................ 3 Coding/ Billing Information ................................... 6 References .......................................................... 6 INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna Companies. Certain Cigna Companies and/or lines of business only provide utilization review services to clients and do not make coverage determinations. References to standard benefit plan language and coverage determinations do not apply to those clients. Coverage Policies are intended to provide guidance in interpreting certain standard benefit plans administered by Cigna Companies. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies -

Amphetamine/Dextroamphetamine IR Generic
GEORGIA MEDICAID FEE-FOR-SERVICE STIMULANT AND RELATED AGENTS PA SUMMARY Preferred Non-Preferred Amphetamine/dextroamphetamine IR generic Adzenys ER (amphetamine ER oral suspension) Armodafinil generic Adzenys XR (amphetamine ER dispersible tab) Atomoxetine generic Amphetamine/dextroamphetamine ER (generic Concerta (methylphenidate ER/SA) Adderall XR) Dextroamphetamine IR tablets generic Aptensio XR (methylphenidate ER) Focalin (dexmethylphenidate) Clonidine ER generic Focalin XR (dexmethylphenidate ER) Cotempla XR (methylphenidate ER disintegrating Guanfacine ER generic tablet) Methylin oral solution (methylphenidate) Daytrana (methylphenidate TD patch) Methylphenidate CD/CR/ER generic by Lannett Desoxyn (methamphetamine) [NDCs 00527-####-##] and Kremers Urban [NDCs Dexmethylphenidate IR generic 62175-####-##] (generic Metadate CD) Dexmethylphenidate ER generic Methylphenidate IR generic Dextroamphetamine ER capsules generic Modafinil generic Dextroamphetamine oral solution generic Quillichew ER (methylphenidate ER chew tabs) Dyanavel XR (amphetamine ER oral suspension) Quillivant XR (methylphenidate ER oral suspension) Evekeo (amphetamine tablets) Vyvanse (lisdexamfetamine) Methamphetamine generic Zenzedi 5 mg, 10 mg IR tablets (dextroamphetamine) Methylphenidate IR chewable tablets generic Methylphenidate ER/SA (generic Concerta) Methylphenidate ER/LA/SR (generic Ritalin LA, Ritalin SR, Metadate ER) Methylphenidate ER/SA 72 mg generic Methylphenidate oral solution generic Mydayis (amphetamine/dextroamphetamine ER) Ritalin LA 10 mg -

MEDICATION GUIDE Armodafinil Tablets, C-IV Read the Medication Guide That Comes with Armodafinil Before You Start Taking It and Each Time You Get a Refill
MEDICATION GUIDE Armodafinil Tablets, C-IV Read the Medication Guide that comes with armodafinil before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your doctor about your condition or treatment. What is the most important information I should know about armodafinil? Armodafinil may cause serious side effects including a serious rash or a serious allergic reaction that may affect parts of your body such as your liver or blood cells. Any of these may need to be treated in a hospital and may be life-threatening. Stop taking armodafinil and call your doctor right away or get emergency help if you have any of these symptoms: • skin rash, hives, sores in your mouth, or your skin blisters and peels • swelling of your face, eyes, lips, tongue, or throat • trouble swallowing or breathing • fever, shortness of breath, swelling of the legs, yellowing of the skin or whites of the eyes, or dark urine. If you have a severe rash with armodafinil, stopping the medicine may not keep the rash from becoming life- threatening or causing you to be permanently disabled or disfigured. Armodafinil is not approved for use in children for any medical condition. It is not known if armodafinil is safe or if it works in children under the age of 17. What is armodafinil? Armodafinil is a prescription medicine used to improve wakefulness in adults who are very sleepy due to one of the following diagnosed sleep disorders: • narcolepsy • obstructive sleep apnea (OSA). -

2010 Buenos Aires, Argentina
Claiming CME Credit To claim CME credit for your participation in the MDS 14th Credit Designation International Congress of Parkinson’s Disease and Movement The Movement Disorder Society designates this educational Disorders, International Congress participants must complete activity for a maximum of 35 AMA PRA Category 1 Credits™. and submit an online CME Request Form. This form will be Physicians should only claim credit commensurate with the available beginning June 15. extent of their participation in the activity. Instructions for claiming credit: If you need a Non-CME Certificate of Attendance, please tear • After June 15, visit the MDS Web site. out the Certificate in the back of this Program and write in • Log in after reading the instructions on the page. You will your name. need your International Congress File Number which is located on your name badge or e-mail The Movement Disorder Society has sought accreditation from [email protected]. the European Accreditation Council for Continuing Medical • Follow the on-screen instructions to claim CME Credit for Education (EACCME) to provide CME activity for medical the sessions you attended. specialists. The EACCME is an institution of the European • You may print your certificate from your home or office, or Union of Medical Specialists (UEMS). For more information, save it as a PDF for your records. visit the Web site: www.uems.net. Continuing Medical Education EACCME credits are recognized by the American Medical The Movement Disorder Society is accredited by the Association towards the Physician’s Recognition Award (PRA). Accreditation Council for Continuing Medical Education To convert EACCME credit to AMA PRA category 1 credit, (ACCME) to provide continuing medical education for contact the AMA online at www.ama-assn.org. -

Stimulant and Related Medications: US Food and Drug
Stimulant and Related Medications: U.S. Food and Drug Administration-Approved Indications and Dosages for Use in Adults The therapeutic dosing recommendations for stimulant and related medications are based on U.S. Food and Drug Administration (FDA)-approved product labeling. Nevertheless, the dosing regimen is adjusted according to a patient’s individual response to pharmacotherapy. The FDA-approved dosages and indications for the use of stimulant and related medications in adults are provided in this table. All medication doses listed are for oral administration. Information on the generic availability of the stimulant and related medications can be found by searching the Electronic Orange Book at https://www.accessdata.fda.gov/scripts/cder/ob/default.cfm on the FDA website. Generic Medication Indication Dosing Information Other Information Availability amphetamine/dextroamphetamine ADHD Initial dose: May increase daily dose by 5 mg at Yes mixed salts[1] 5 mg once or twice a day; weekly intervals until optimal response Maximum dose: 40 mg per day is achieved. Only in rare cases will it be necessary to exceed a total of 40 mg per day. amphetamine/dextroamphetamine narcolepsy Initial dose: 10 mg per day; May increase daily dose by 10 mg at Yes mixed salts Usual dose: weekly intervals until optimal response 5 mg to 60 mg per day is achieved. Take first dose in divided doses upon awakening. amphetamine/dextroamphetamine ADHD Recommended dose: Patients switching from regular-release Yes mixed salts ER*[2] 20 mg once a day amphetamine/dextroamphetamine mixed salts may take the same total daily dose once a day. armodafinil[3] narcolepsy Recommended dose: Take as a single dose in the morning. -

Monoamine Reuptake Inhibitors in Parkinson's Disease
Hindawi Publishing Corporation Parkinson’s Disease Volume 2015, Article ID 609428, 71 pages http://dx.doi.org/10.1155/2015/609428 Review Article Monoamine Reuptake Inhibitors in Parkinson’s Disease Philippe Huot,1,2,3 Susan H. Fox,1,2 and Jonathan M. Brotchie1 1 Toronto Western Research Institute, Toronto Western Hospital, University Health Network, 399 Bathurst Street, Toronto, ON, Canada M5T 2S8 2Division of Neurology, Movement Disorder Clinic, Toronto Western Hospital, University Health Network, University of Toronto, 399BathurstStreet,Toronto,ON,CanadaM5T2S8 3Department of Pharmacology and Division of Neurology, Faculty of Medicine, UniversitedeMontr´ eal´ and Centre Hospitalier de l’UniversitedeMontr´ eal,´ Montreal,´ QC, Canada Correspondence should be addressed to Jonathan M. Brotchie; [email protected] Received 19 September 2014; Accepted 26 December 2014 Academic Editor: Maral M. Mouradian Copyright © 2015 Philippe Huot et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The motor manifestations of Parkinson’s disease (PD) are secondary to a dopamine deficiency in the striatum. However, the degenerative process in PD is not limited to the dopaminergic system and also affects serotonergic and noradrenergic neurons. Because they can increase monoamine levels throughout the brain, monoamine reuptake inhibitors (MAUIs) represent potential therapeutic agents in PD. However, they are seldom used in clinical practice other than as antidepressants and wake-promoting agents. This review article summarises all of the available literature on use of 50 MAUIs in PD. The compounds are divided according to their relative potency for each of the monoamine transporters. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub
US 20090005722A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0005722 A1 Jennings-Spring (43) Pub. Date: Jan. 1, 2009 (54) SKIN-CONTACTING-ADHESIVE FREE Publication Classification DRESSING (51) Int. Cl. Inventor: Barbara Jennings-Spring, Jupiter, A61N L/30 (2006.01) (76) A6F I3/00 (2006.01) FL (US) A6IL I5/00 (2006.01) Correspondence Address: AOIG 7/06 (2006.01) Irving M. Fishman AOIG 7/04 (2006.01) c/o Cohen, Tauber, Spievack and Wagner (52) U.S. Cl. .................. 604/20: 602/43: 602/48; 4771.5; Suite 2400, 420 Lexington Avenue 47/13 New York, NY 10170 (US) (57) ABSTRACT (21) Appl. No.: 12/231,104 A dressing having a flexible sleeve shaped to accommodate a Substantially cylindrical body portion, the sleeve having a (22) Filed: Aug. 29, 2008 lining which is substantially non-adherent to the body part being bandaged and having a peripheral securement means Related U.S. Application Data which attaches two peripheral portions to each other without (63) Continuation-in-part of application No. 1 1/434,689, those portions being circumferentially adhered to the sleeve filed on May 16, 2006. portion. Patent Application Publication Jan. 1, 2009 Sheet 1 of 9 US 2009/0005722 A1 Patent Application Publication Jan. 1, 2009 Sheet 2 of 9 US 2009/0005722 A1 10 8 F.G. 5 Patent Application Publication Jan. 1, 2009 Sheet 3 of 9 US 2009/0005722 A1 13 FIG.6 2 - Y TIII Till "T fift 11 10 FIG.7 8 13 6 - 12 - Timir" "in "in "MINIII. -

Sodium Oxybate (Xyrem®) Armodafinil (Nuvigil®) Solriamfetol (Sunosi®) Approval December 1998 July 2002 June 2007 March 2019 Cephalon, Inc
Modafanil (Provigil®) Sodium Oxybate (Xyrem®) Armodafinil (Nuvigil®) Solriamfetol (Sunosi®) Approval December 1998 July 2002 June 2007 March 2019 Cephalon, Inc. Orpahn Medical Cephalon, Inc. Jazz Pharmaceuticals Schedule C‐IV C‐III C‐IV C‐IV Sodium salt of gamma hydroxybutyrate (GHB active ingredient, Schedule I) Indication To improve wakefulness in adults with Treatment of cataplexy or excessive daytime To improve wakefulness in adults with To improve wakefulness in adults with excessive sleepiness associated with sleepiness in patients with Narcolepsy excessive sleepiness associated with excessive daytime sleepiness associated with narcolepsy, OSA, or shift work disorder narcolepsy, OSA, or shift work disorder narcolepsy or OSA (underlying airway obstruction should be treated for at least 1 month prior to & during Solriamfetol therapy Non-FDA Uses ● ADHD ● MDD (antidepressant ●Alcoholism ● Alcohol withdrawal syndrome ● Bipolar disorder, depressed phase, in - augmentation) ● Depression Unipolar or ● Breathing-related sleep disorder ● combination with conventional medications bipolar (adjunct) Fibromyalgia ● General anesthesia ● Opioid ● Multiple sclerosis/Depression withdrawal ● Sedation (adjunct)/Cancer related fatigue ● Schizophrenia (adjunct) ● Sleep deprivation ● Spastic cerebral palsy ● Steinert myotonic dystrophy syndrome MOA ●Modafinil increases dopamine by blocking Sodium oxybate’s (a CNS depressant) effects Armodafinil, the R-enantiomer of modafinil, Selective dopamine and norepinephrine (unclear) dopamine transporters are thought -

Review Article Monoamine Reuptake Inhibitors in Parkinson's Disease
Review Article Monoamine Reuptake Inhibitors in Parkinson’s Disease Philippe Huot,1,2,3 Susan H. Fox,1,2 and Jonathan M. Brotchie1 1 Toronto Western Research Institute, Toronto Western Hospital, University Health Network, 399 Bathurst Street, Toronto, ON, Canada M5T 2S8 2Division of Neurology, Movement Disorder Clinic, Toronto Western Hospital, University Health Network, University of Toronto, 399BathurstStreet,Toronto,ON,CanadaM5T2S8 3Department of Pharmacology and Division of Neurology, Faculty of Medicine, UniversitedeMontr´ eal´ and Centre Hospitalier de l’UniversitedeMontr´ eal,´ Montreal,´ QC, Canada Correspondence should be addressed to Jonathan M. Brotchie; [email protected] Received 19 September 2014; Accepted 26 December 2014 Academic Editor: Maral M. Mouradian Copyright © 2015 Philippe Huot et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. The motor manifestations of Parkinson’s disease (PD) are secondary to a dopamine deficiency in the striatum. However, the degenerative process in PD is not limited to the dopaminergic system and also affects serotonergic and noradrenergic neurons. Because they can increase monoamine levels throughout the brain, monoamine reuptake inhibitors (MAUIs) represent potential therapeutic agents in PD. However, they are seldom used in clinical practice other than as antidepressants and wake-promoting agents. This review -

World of Cognitive Enhancers
ORIGINAL RESEARCH published: 11 September 2020 doi: 10.3389/fpsyt.2020.546796 The Psychonauts’ World of Cognitive Enhancers Flavia Napoletano 1,2, Fabrizio Schifano 2*, John Martin Corkery 2, Amira Guirguis 2,3, Davide Arillotta 2,4, Caroline Zangani 2,5 and Alessandro Vento 6,7,8 1 Department of Mental Health, Homerton University Hospital, East London Foundation Trust, London, United Kingdom, 2 Psychopharmacology, Drug Misuse, and Novel Psychoactive Substances Research Unit, School of Life and Medical Sciences, University of Hertfordshire, Hatfield, United Kingdom, 3 Swansea University Medical School, Institute of Life Sciences 2, Swansea University, Swansea, United Kingdom, 4 Psychiatry Unit, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy, 5 Department of Health Sciences, University of Milan, Milan, Italy, 6 Department of Mental Health, Addictions’ Observatory (ODDPSS), Rome, Italy, 7 Department of Mental Health, Guglielmo Marconi” University, Rome, Italy, 8 Department of Mental Health, ASL Roma 2, Rome, Italy Background: There is growing availability of novel psychoactive substances (NPS), including cognitive enhancers (CEs) which can be used in the treatment of certain mental health disorders. While treating cognitive deficit symptoms in neuropsychiatric or neurodegenerative disorders using CEs might have significant benefits for patients, the increasing recreational use of these substances by healthy individuals raises many clinical, medico-legal, and ethical issues. Moreover, it has become very challenging for clinicians to Edited by: keep up-to-date with CEs currently available as comprehensive official lists do not exist. Simona Pichini, Methods: Using a web crawler (NPSfinder®), the present study aimed at assessing National Institute of Health (ISS), Italy Reviewed by: psychonaut fora/platforms to better understand the online situation regarding CEs. -
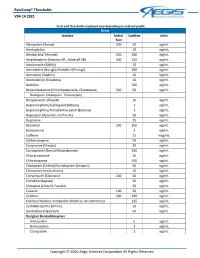
Paincomp® Thresholds V04.14.2021 Copyright © 2020 Aegis Sciences
PainComp® Thresholds V04.14.2021 Tests and thresholds employed vary depending on ordered profile Urine Analyte Initial Confirm Units Test Alprazolam (Xanax) 200 50 ng/mL Amitriptyline 10 ng/mL Amobarbital (Amytal) 200 200 ng/mL Amphetamine (Adzenys ER , Adderall XR) 500 250 ng/mL Aripiprazole (Abilify) 10 ng/mL Armodafinil (Nuvigil)/Modafinil (Provigil) 100 ng/mL Asenapine (Saphris) 10 ng/mL Atomoxetine (Strattera) 10 ng/mL Baclofen 500 ng/mL Benzodiazepines (Chlordiazepoxide, Clorazepate, 200 50 ng/mL Diazepam, Oxazepam, Temazepam) Brexpiprazole (Rexulti) 10 ng/mL Buprenorphine/Sublingual (Belbuca) 1 ng/mL Buprenorphine/Transdermal patch (Butrans) 1 ng/mL Bupropion (Aplenzin, Forfivo XL) 50 ng/mL Buspirone 25 ng/mL Butalbital 200 200 ng/mL Butorphanol 1 ng/mL Caffeine 15 mcg/mL Carbamazepine 50 ng/mL Cariprazine (Vraylar) 20 ng/mL Carisoprodol (Soma)/Meprobamate 200 ng/mL Chlorpromazine 10 ng/mL Chlorzoxazone 500 ng/mL Citalopram (Celexa)/Escitalopram (Lexapro) 50 ng/mL Clomipramine (Anafranil) 10 ng/mL Clonazepam (Klonopin) 200 50 ng/mL Clonidine (Kapvay) 50 ng/mL Clozapine (Clozaril, Fazaclo) 50 ng/mL Cocaine 100 50 ng/mL Codeine 200 100 ng/mL Cotinine/Nicotine metabolite (Habitrol, Nicoderm CQ) 125 ng/mL Cyclobenzaprine (Amrix) 10 ng/mL Dantrolene (Dantrium) 50 ng/mL Designer Benzodiazepines Adinazolam 5 ng/mL Bromazolam 1 ng/mL Clonazolam 1 ng/mL Copyright © 2020 Aegis Sciences Corporation All Rights Reserved PainComp® Thresholds V04.14.2021 Deschloroetizolam 1 ng/mL Diclazepam 1 ng/mL Etizolam 1 ng/mL Flualprazolam 1 ng/mL Flubromazepam -

FDA Approved Labeling Text for NDA 21-875/NUVIGIL™ (Armodafinil) Tablets Approved Labeling Dated June 15, 2007
FDA Approved Labeling Text for NDA 21-875/NUVIGIL™ (armodafinil) Tablets Approved Labeling dated June 15, 2007 1 NUVIGIL™ (armodafinil) Tablets [C-IV] Rx Only 2 3 4 5 DESCRIPTION 6 NUVIGILTM (armodafinil) is a wakefulness-promoting agent for oral administration. 7 Armodafinil is the R-enantiomer of modafinil which is a mixture of the R- and S- 8 enantiomers. The chemical name for armodafinil is 2-[(R)- 9 (diphenylmethyl)sulfinyl]acetamide. The molecular formula is C15H15NO2S and the 10 molecular weight is 273.35. 11 12 The chemical structure is: O O S NH2 13 14 15 Armodafinil is a white to off-white, crystalline powder that is very slightly soluble in 16 water, sparingly soluble in acetone and soluble in methanol. NUVIGIL tablets contain 17 50, 150 or 250 mg of armodafinil and the following inactive ingredients: croscarmellose 18 sodium, lactose, magnesium stearate, microcrystalline cellulose, povidone, and 19 pregelatinized starch. 20 21 CLINICAL PHARMACOLOGY 22 Mechanism of Action and Pharmacology 23 The precise mechanism(s) through which armodafinil (R-enantiomer) or modafinil 24 (mixture of R- and S-enantiomers) promote wakefulness is unknown. Both armodafinil 25 and modafinil have shown similar pharmacological properties in nonclinical animal and 26 in vitro studies, to the extent tested. 27 1 FDA Approved Labeling Text for NDA 21-875/NUVIGIL™ (armodafinil) Tablets Approved Labeling dated June 15, 2007 28 At pharmacologically relevant concentrations, armodafinil does not bind to or inhibit 29 several receptors and enzymes potentially relevant for sleep/wake regulation, including 30 those for serotonin, dopamine, adenosine, galanin, melatonin, melanocortin, orexin-1, 31 orphanin, PACAP or benzodiazepines, or transporters for GABA, serotonin, 32 norepinephrine, and choline or phosphodiesterase VI, COMT, GABA transaminase, and 33 tyrosine hydroxylase.