Background Paper 6.4 Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Basis of Blood Glucose Regulation Asma Ahmed and Noman Khalique
Chapter Molecular Basis of Blood Glucose Regulation Asma Ahmed and Noman Khalique Abstract Blood glucose level is regulated by multiple pancreatic hormones, which regulate it by different pathways in normal and abnormal conditions by expressing or suppressing multiple genes or molecular or cellular targets. Multiple synthetic drugs and therapies are used to cure glucose regulatory problems, while many of them are used to cure other health issues, which arise due to disturbance in blood glucose regulations. Many new approaches are used for the development of phytochemical- based drugs to cure blood glucose regulation problems, and many of the compounds have been isolated and identified to cure insulin resistance or regulate beta cell function or glucose absorption in the guts or GLP-1 homoeostasis or two/more pathways (e.g., either cure hyperglycemia or raise insulin resistance or cure pan- creatic beta cell regeneration or augmentation of GLP-1, production of islet cell, production and increased insulin receptor signaling and insulin secretion or decreased insulin tolerance or gluconeogenesis and insulin-mimetic action or pro- duction of α-glucosidase and α-amylase inhibitor or conserve islet mass or activate protein kinase A (PKA) and extracellular signal regulated kinases (ERK) or activate AMPK and reduce insulin sensitivity or suppress α-glucosidase activity and activate AMPK and downstream molecules or prevents cell death of pancreatic β-cell and activates SIRT1 or lower blood glucose due to their insulin-like chemical structures or decrease lipid peroxidation. Keywords: genes, molecular and cellular targets, hormones, pathways 1. Introduction Blood glucose is regulated by the pancreatic hormones alone or in combination with other endocrine glands and all this is controlled by one or more gene or cellular or molecular targets. -

Expect the Unexpected: Building Business Value in a Changing World
Expect the Unexpected: Building business value in a changing world kpmg.com KPMG INTERNATIONAL PART 1 b | Expect the Unexpected: Building business value in a changing world In this report we offer a starting point for discussion. We present a system of ten sustainability megaforces that will impact each and every business over the next 20 years. We want to build awareness that these forces do not act alone in predictable ways. They are interconnected. They interact. Disclaimer: Throughout this document, "KPMG" ["we," "our," and "us"] refers to KPMG International, a Swiss entity that serves as a coordinating entity for a network of independent member firms operating under the KPMG name, KPMG's Climate Change and Sustainability practice, and/or to any one or more of such firms. KPMG International provides no client services. © 2012 KPMG International Cooperative (“KPMG International”), a Swiss entity. Member firms of the KPMG network of independent firms are affiliated with KPMG International. KPMG International provides no client services. All rights reserved. Expect the Unexpected: Building business value in a changing world | c Foreword usinesses today are operating in an ever more At KPMG’s network of firms we have always been at the interconnected and globalized world. Supply chains forefront of developments that shape business behavior. Bstretch across continents and are vulnerable to We are working with organizations to help them understand disruption. Consumer demands and government policies the forces at work that will influence markets and impact are changing rapidly and will impact your bottom line if your profitability in the medium to long term. -

Novartis Swaps Two Avexis Executives for One Following
No. 3969 August 23, 2019 and CSO that the Kaspars have not been involved in the Novartis subsidiary’s op- erations since early May. Asked whether more AveXis employees will exit the company due to the preclini- cal safety data manipulation revealed on 6 August or whether other actions will be taken by Novartis in relation to the matter, Althoff told Scrip: “We cannot comment on the specifics of who is involved, but we can confirm this issue was not widespread, ei- ther in our overall dataset nor in our com- pany or any function within our company and was limited to a few individuals.” Novartis CEO Vas Narasimhan said in a 7 August conference call about the Zol- gensma data controversy that the compa- ny was in the process of “exiting the small number of AveXis scientists involved” in the data manipulation, which occurred Novartis Swaps Two AveXis before Novartis bought the gene therapy firm. (Also see “Novartis CEO Explains De- lay In Telling US FDA About Zolgensma Executives For One Following Data Fraud: We Wanted To Understand It First” - Pink Sheet, 7 Aug, 2019.) Zolgensma Data Manipulation Novartis acquired AveXis for $8.7bn MANDY JACKSON [email protected] last year. (Also see “Novartis Goes Big On Gene Therapy With $8.7bn AveXis Acquisi- ovartis AG said when it disclosed the Zolgensma BLA was manipulated. Page tion” - Scrip, 9 Apr, 2018.) Zolgensma was that preclinical safety data for Zol- Bouchard, whose most recent function was the lead asset in AveXis’s R&D pipeline and Ngensma were manipulated in the bi- global head of preclinical safety for Novar- won US Food and Drug Administration ologic license application for the gene ther- tis Institutes of Biomedical Research (NIBR), approval in May, becoming the world’s apy that the company would be “exiting” was named senior vice president and chief most expensive drug with a $2m list price. -

Inhaled Insulin: the Solution for Suboptimal Diabetes Control?
I EDITORIALS Inhaled insulin: the solution for suboptimal diabetes control? Stephanie A Amiel Stephanie A Amiel For nearly a century, we have been using insulin to action comparable to human soluble (regular) BSc MD FRCP, treat diabetes. Until recently, that insulin has been insulin rather than fast-acting analogues.10 In trials, RD Lawrence given by injection. In 2006, the first inhaled insulin, Exubera® is only ‘non-inferior’ to conventional (not Professor of Diabetic Exubera®, was licenced for use in Europe. The man- analogue) insulins, the latter not always used in Medicine, King’s ufacturers hoped for a warm welcome – the end of equivalent basal-bolus regimens11–14 and in type 2, College London School of Medicine injections and the dawn of a new era of enthusiastic slightly more effective than adding a second oral insulin takers with perfect diabetic control. The agent in very poorly controlled disease.15,16 It has not Clin Med National Institute for Health and Clinical Excellence been tested against fully flexible analogue regimens. 2007;7:430–2 (NICE) was more cautious and recommend that It is clear that inhaled insulin’s sole advantage over inhaled insulin only be used in the presence of diag- injected insulin is its route of delivery. nosed needle phobia (or severe intractable problems Exubera® has low bioavailability, minor and prob- with injection sites) and should be supervised by dia- ably reversible effects on lung function17 and a nat- betes specialists.1 True needle phobia is rare and ural lack of long-term safety data. Active smoking treatable by standard psychological techniques.2,3 On contraindicates its use because of increased bioavail- the NICE criteria, the market for inhaled insulin may abilty of inhaled insulin,18,19 while absorption may be small indeed. -
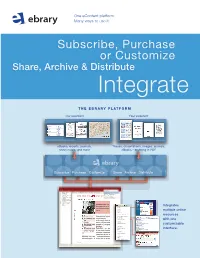
The EBRARY Platform
One eContent platform. Many ways to use it. Subscribe, Purchase or Customize Share, Archive & Distribute Integrate THE EBRARY PLatFORM Our eContent Your eContent eBooks, reports, journals, Theses, dissertations, images, journals, sheet music, and more eBooks – anything in PDF Subscribe Purchase Customize Share Archive Distribute SULAIR Select Collections SUL EBRARY COLLECTIONS Select Collections TABLE OF CONTENTS Infotools All ebrary Collections CONTRIBUTORS Byron Hoyt Sheet Music (Browse) INTRODUCTION Immigration Commision Reports (Dillingham) Contents BUSINESS: A USER’S GUIDE Women and Child Wage Earners in the U.S. CSLI Linguistics and Philosophy BEST PRACTICE SUL Books in the Public Domain MANAGEMENT CHECKLISTS Medieval and Modern Thought Text Project ACTIONLISTS MANAGEMENT LIBRARY BUSINESS THINKERS AND MANA DICTIONARY WORLD BUSINESS ALMANAC Highlights Notes BUSINESS INFORMATION SOURC INDEX CREDITS InfoTools Integrates Define Explain Locate multiple online Translate Search Document... resources Search All Documents... Search Web Search Catalog with one Highlight Add to Bookshelf customizable Copy Text... Copy Bookmark interface. Print... Print Again Toggle Automenu Preferences... Help About ebrary Reader... EASY TO USE. Subscribe, Purchase AFFORDABLE. or Customize your ALW ay S AVAILABLE. eBook selection NO CHECK-OUTS. Un I Q U E S U B SC R I B E T O G R OWI ng E B O O K D ataba S E S W I T H SIMULtanEOUS, MULTI-USER ACCESS. RESEARCH TOOLS. ACADEMIC DATABASES Academic Complete includes all academic databases listed below. FREE MARCS. # of # of Subject Titles* Subject Titles* Business & Economics 6,300 Language, Literature 3,400 & Linguistics “ebrary’s content is Computers & IT 2,800 Law, International Relations 3,800 multidisciplinary and Education 2,300 & Public Policy Engineering & Technology 3,700 supports our expanding Life Sciences 2,000 (includes Biotechnolgy, History & Political Science 4,800 Agriculture, and (also includes a bonus selection faculty and curriculum. -

Annual Copyright License for Corporations
Responsive Rights - Annual Copyright License for Corporations Publisher list Specific terms may apply to some publishers or works. Please refer to the Annual Copyright License search page at http://www.copyright.com/ to verify publisher participation and title coverage, as well as any applicable terms. Participating publishers include: Advance Central Services Advanstar Communications Inc. 1105 Media, Inc. Advantage Business Media 5m Publishing Advertising Educational Foundation A. M. Best Company AEGIS Publications LLC AACC International Aerospace Medical Associates AACE-Assn. for the Advancement of Computing in Education AFCHRON (African American Chronicles) AACECORP, Inc. African Studies Association AAIDD Agate Publishing Aavia Publishing, LLC Agence-France Presse ABC-CLIO Agricultural History Society ABDO Publishing Group AHC Media LLC Abingdon Press Ahmed Owolabi Adesanya Academy of General Dentistry Aidan Meehan Academy of Management (NY) Airforce Historical Foundation Academy Of Political Science Akademiai Kiado Access Intelligence Alan Guttmacher Institute ACM (Association for Computing Machinery) Albany Law Review of Albany Law School Acta Ecologica Sinica Alcohol Research Documentation, Inc. Acta Oceanologica Sinica Algora Publishing ACTA Publications Allerton Press Inc. Acta Scientiae Circumstantiae Allied Academies Inc. ACU Press Allied Media LLC Adenine Press Allured Business Media Adis Data Information Bv Allworth Press / Skyhorse Publishing Inc Administrative Science Quarterly AlphaMed Press 9/8/2015 AltaMira Press American -

Global Business Information Specialist Informa’S Book Business Has More Than 55,000 Academic Advisers 26 Directors’ Report 27 and Business Titles
Business Profile Business WHO WE ARE Business Profile 02 Annual Report & Financial Statements 2009 Our Business 03 Informa at a Glance 04 Academic Information 06 Professional & Commercial Information 08 Events & Training 10 Informa plc is a leading international provider of specialist information and services for the academic and scientific, professional and commercial business communities. Informa has some 150 offices in over 40 countries and employs approximately 8,000 staff around the world. Informa is the largest publicly-owned organiser of conferences and courses in the world with inThe Year The Year in Review 12 an output of around 8,000 events annually. Informa Financial Highlights 13 publishes over 2,100 subscription-based information Chairman’s Statement 14 services including academic journals, real-time news Chief Executive’s Review 16 Financial Review 19 R and structured databases of commercial intelligence. Board of Directors 24 eview Global Business Information Specialist Informa’s book business has more than 55,000 academic Advisers 26 Directors’ Report 27 and business titles. Corporate Governance Statement 38 Directors’ Remuneration Report 45 Corporate Responsibility 55 Financial Statements Financial Financial Statements 58 Independent Auditors’ Report – Group 59 Consolidated Income Statement 60 Consolidated Statement of Comprehensive Income 61 Consolidated Statement of Changes in Equity 62 Consolidated Statement of Financial Position 64 ”It was an extremely demanding year, Consolidated Cash Flow Statement 65 but one which -

The Un-Design and Design of Insulin: Structural Evolution
THE UN-DESIGN AND DESIGN OF INSULIN: STRUCTURAL EVOLUTION WITH APPLICATION TO THERAPEUTIC DESIGN By NISCHAY K. REGE Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Department of Biochemistry Dissertation Advisor: Dr. Michael A. Weiss CASE WESTERN RESERVE UNIVERSITY August, 2018 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Nischay K. Rege candidate for the degree of Doctor of Philosophy*. Committee Chair Paul Carey Committee Member Michael Weiss Committee Member Faramarz Ismail-Beigi Committee Member George Dubyak Date of Defense: June 26th, 2018 *We also certify that written approval has been obtained for any proprietary material contained therein. Dedication This thesis is dedicated to my mother, Dipti, whose constant love and faith have never failed, to my father, Kiran, who taught me of the virtue of curiosity, and to my wife, Shipra, whose kindness and companionship have given me enough strength for eight lifetimes. i Table of Contents Dedication ..................................................................................................................................... i Table of Contents ......................................................................................................................... ii List of Tables ............................................................................................................................... v List of Figures ........................................................................................................................... -

Informa Corporate Structure
Corporate Structure From www.informa.com/Who-We-Are/Corporate-structure/ 5 March 2011 History Edward Lloyd pins the first ever copy of his shipping list to the wall of his coffee shop 1734 in Lombard Street, City of London. Richard Taylor publishes the first edition of The Philosophical Magazine, one of the 1798 first scientific journals produced by an independent company Dr William Francis joined Richard Taylor to found Taylor & Francis and continue the 1852 close links between the academic community and the company Taylor & Francis became a private limited company with leading scientists as 1936 directors and shareholders 1964 Investment & Property Studies founded as a part-time business International Business Communications Ltd (IBC) created to be an umbrella company 1971 for Investment & Property Studies and Legal Studies & Services The Institute for International Research (IIR) is launched by Irvine Laidlaw later to 1973 become Baron Laidlaw of Rothiemay – IIR becomes a specialist business conference organiser 1976 Euroforum established in Holland by IBC 1985 International Business Communications (Holdings) plc established Datamonitor is founded in London and produces its first ever report on the UK frozen 1989 food industry 1991 Launch of The Monaco Yacht Show by IIR Lloyd's List Publishing (LLP) and IBC Group plc merge to form the Informa Group 1998 plc Taylor & Francis successfully launched on the London Stock Exchange and shortly 1998 afterwards acquired the Routledge Group of companies Datamonitor, now a world-leading provider -

Maikawa STM 2021.Pdf
SCIENCE TRANSLATIONAL MEDICINE | REVIEW DRUG DEVELOPMENT Copyright © 2021 The Authors, some rights reserved; Engineering biopharmaceutical formulations exclusive licensee American Association to improve diabetes management for the Advancement Caitlin L. Maikawa1, Andrea I. d’Aquino2, Rayhan A. Lal3,4,5, of Science. No claim 4,5 1,2,4,5,6 to original U.S. Bruce A. Buckingham , Eric A. Appel * Government Works Insulin was first isolated almost a century ago, yet commercial formulations of insulin and its analogs for hormone replacement therapy still fall short of appropriately mimicking endogenous glycemic control. Moreover, the controlled delivery of complementary hormones (such as amylin or glucagon) is complicated by instability of the pharmacologic agents and complexity of maintaining multiple infusions. In this review, we highlight the advantages and limitations of recent advances in drug formulation that improve protein stability and pharmacokinetics, prolong drug delivery, or enable alternative dosage forms for the management of diabetes. With controlled delivery, Downloaded from these formulations could improve closed-loop glycemic control. ENGINEERING OPPORTUNITIES TO IMPROVE DIABETES tive treatment with insulin alone is already highly burdensome and MANAGEMENT costly—requiring frequent glucose monitoring, mealtime insulin Over the past century, insulin replacement therapy has been impera- boluses, basal insulin delivery through infusion pumps or long-acting http://stm.sciencemag.org/ tive to saving lives and improving diabetes treatment outcomes. The analogs, and routine carbohydrate counting—and still does not truly administration of exogenous insulin prevents ketoacidosis, which recapitulate the complexities of metabolic control in nondiabetic was once a universally fatal condition. Although insulin has histor- individuals (Fig. 1B) (7). -

The Leading Source of Diabetes Business News It's Complicated
The Leading Source of Diabetes Business News It’s Complicated January/February 2011 • No. 105 The diabetes business used to be fairly straightforward. It’s a lot more complicated now, but that’s not a bad thing. Take insulin. After it was commercialized in the early 1920s, the goal was clear: sell more insulin. But look at today’s three major insulin makers: Eli Lilly, sanofi-aventis, and Novo Nordisk. For all three, to be sure, insulin is still preeminent. The diabetes divisions of Novo Nordisk and Lilly draw most of their revenue from human insulin and rapid-acting analogs, while sanofi-aventis’ blockbuster basal analog Lantus, as well as its mealtime insulins, continue to drive the bottom line. But all three companies have also developed (or are developing) GLP-1 analogs, Lilly has invested in oral drugs for type 2 diabetes (most recently through a major partnership with Boehringer Ingelheim), and the companies are also developing new therapeutic approaches for different stages of disease progression. The evolution of the insulin makers reflects broader industry trends. As our understanding of diabetes has expanded – and we dig deeper into the molecular complexities of the disease – it’s clear that single- focus large companies can no longer prosper. Ours is an era of individualized treatment that mixes and matches different drugs and technologies that try to meet the unique needs of every person with diabetes. Toward that end, companies are forming partnerships to find complementary therapies and development efforts that will create -

Various Emerging Trends in Insulin Drug Delivery Systems
British Journal of Pharmaceutical Research 5(5): 294-308, 2015, Article no.BJPR.2015.029 ISSN: 2231-2919 SCIENCEDOMAIN international www.sciencedomain.org Various Emerging Trends in Insulin Drug Delivery Systems Yasmeen1, T. Mamatha1*, Md. Zubair1, Sana Begum1 and Tayyaba Muneera1 1Department of Pharmaceutics, Sultan-ul-Uloom College of Pharmacy, affiliated to Jawaharlal Nehru Technological University Hyderabad, Road No.3, Banjara hills, Hyderabad 500 034, Telangana, India. Authors’ contributions This work was carried out in collaboration between all authors. Author TM designed the study, Authors Y and SB wrote the protocol, and wrote the first draft of the manuscript. Authors Md. Z and TM managed the literature searches. All authors read and approved the final manuscript. Article Information DOI:10.9734/BJPR/2015/12528 Editor(s): (1) Rafik Karaman, Bioorganic Chemistry, College of Pharmacy, Al-Quds University, USA. (2) Alyautdin Renad N, The Department of Pharmacology, I. M. Sechenov MSMU, Russia. Reviewers: (1) Anonymous, Italy. (2) Anonymous, Spain. (3) Anonymous, Norway. (4) Wei Wu, School of Pharmacy, Fudan University, China. (5) Anonymous, Malaysia. (6) Anastasia Thanopoulou, Diabetes Centre and Department of Internal Medicine, National University of Athens, Hippokration General Hospital, Athens, Greece. Complete Peer review History: http://www.sciencedomain.org/review-history.php?iid=883&id=14&aid=7718 Received 4th July 2014 th Review Article Accepted 28 November 2014 Published 9th January 2015 ABSTRACT Lowering of blood glucose level in patients can be achieved by insulin therapy as it plays a key role in the control of hyperglycaemia for type 1 diabetes. Insulin delivery systems that are currently available include syringes, infusion pumps, jet injectors and pens.