Novartis Swaps Two Avexis Executives for One Following
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Expect the Unexpected: Building Business Value in a Changing World
Expect the Unexpected: Building business value in a changing world kpmg.com KPMG INTERNATIONAL PART 1 b | Expect the Unexpected: Building business value in a changing world In this report we offer a starting point for discussion. We present a system of ten sustainability megaforces that will impact each and every business over the next 20 years. We want to build awareness that these forces do not act alone in predictable ways. They are interconnected. They interact. Disclaimer: Throughout this document, "KPMG" ["we," "our," and "us"] refers to KPMG International, a Swiss entity that serves as a coordinating entity for a network of independent member firms operating under the KPMG name, KPMG's Climate Change and Sustainability practice, and/or to any one or more of such firms. KPMG International provides no client services. © 2012 KPMG International Cooperative (“KPMG International”), a Swiss entity. Member firms of the KPMG network of independent firms are affiliated with KPMG International. KPMG International provides no client services. All rights reserved. Expect the Unexpected: Building business value in a changing world | c Foreword usinesses today are operating in an ever more At KPMG’s network of firms we have always been at the interconnected and globalized world. Supply chains forefront of developments that shape business behavior. Bstretch across continents and are vulnerable to We are working with organizations to help them understand disruption. Consumer demands and government policies the forces at work that will influence markets and impact are changing rapidly and will impact your bottom line if your profitability in the medium to long term. -
The EBRARY Platform
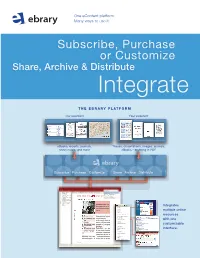
One eContent platform. Many ways to use it. Subscribe, Purchase or Customize Share, Archive & Distribute Integrate THE EBRARY PLatFORM Our eContent Your eContent eBooks, reports, journals, Theses, dissertations, images, journals, sheet music, and more eBooks – anything in PDF Subscribe Purchase Customize Share Archive Distribute SULAIR Select Collections SUL EBRARY COLLECTIONS Select Collections TABLE OF CONTENTS Infotools All ebrary Collections CONTRIBUTORS Byron Hoyt Sheet Music (Browse) INTRODUCTION Immigration Commision Reports (Dillingham) Contents BUSINESS: A USER’S GUIDE Women and Child Wage Earners in the U.S. CSLI Linguistics and Philosophy BEST PRACTICE SUL Books in the Public Domain MANAGEMENT CHECKLISTS Medieval and Modern Thought Text Project ACTIONLISTS MANAGEMENT LIBRARY BUSINESS THINKERS AND MANA DICTIONARY WORLD BUSINESS ALMANAC Highlights Notes BUSINESS INFORMATION SOURC INDEX CREDITS InfoTools Integrates Define Explain Locate multiple online Translate Search Document... resources Search All Documents... Search Web Search Catalog with one Highlight Add to Bookshelf customizable Copy Text... Copy Bookmark interface. Print... Print Again Toggle Automenu Preferences... Help About ebrary Reader... EASY TO USE. Subscribe, Purchase AFFORDABLE. or Customize your ALW ay S AVAILABLE. eBook selection NO CHECK-OUTS. Un I Q U E S U B SC R I B E T O G R OWI ng E B O O K D ataba S E S W I T H SIMULtanEOUS, MULTI-USER ACCESS. RESEARCH TOOLS. ACADEMIC DATABASES Academic Complete includes all academic databases listed below. FREE MARCS. # of # of Subject Titles* Subject Titles* Business & Economics 6,300 Language, Literature 3,400 & Linguistics “ebrary’s content is Computers & IT 2,800 Law, International Relations 3,800 multidisciplinary and Education 2,300 & Public Policy Engineering & Technology 3,700 supports our expanding Life Sciences 2,000 (includes Biotechnolgy, History & Political Science 4,800 Agriculture, and (also includes a bonus selection faculty and curriculum. -

Annual Copyright License for Corporations
Responsive Rights - Annual Copyright License for Corporations Publisher list Specific terms may apply to some publishers or works. Please refer to the Annual Copyright License search page at http://www.copyright.com/ to verify publisher participation and title coverage, as well as any applicable terms. Participating publishers include: Advance Central Services Advanstar Communications Inc. 1105 Media, Inc. Advantage Business Media 5m Publishing Advertising Educational Foundation A. M. Best Company AEGIS Publications LLC AACC International Aerospace Medical Associates AACE-Assn. for the Advancement of Computing in Education AFCHRON (African American Chronicles) AACECORP, Inc. African Studies Association AAIDD Agate Publishing Aavia Publishing, LLC Agence-France Presse ABC-CLIO Agricultural History Society ABDO Publishing Group AHC Media LLC Abingdon Press Ahmed Owolabi Adesanya Academy of General Dentistry Aidan Meehan Academy of Management (NY) Airforce Historical Foundation Academy Of Political Science Akademiai Kiado Access Intelligence Alan Guttmacher Institute ACM (Association for Computing Machinery) Albany Law Review of Albany Law School Acta Ecologica Sinica Alcohol Research Documentation, Inc. Acta Oceanologica Sinica Algora Publishing ACTA Publications Allerton Press Inc. Acta Scientiae Circumstantiae Allied Academies Inc. ACU Press Allied Media LLC Adenine Press Allured Business Media Adis Data Information Bv Allworth Press / Skyhorse Publishing Inc Administrative Science Quarterly AlphaMed Press 9/8/2015 AltaMira Press American -

Global Business Information Specialist Informa’S Book Business Has More Than 55,000 Academic Advisers 26 Directors’ Report 27 and Business Titles
Business Profile Business WHO WE ARE Business Profile 02 Annual Report & Financial Statements 2009 Our Business 03 Informa at a Glance 04 Academic Information 06 Professional & Commercial Information 08 Events & Training 10 Informa plc is a leading international provider of specialist information and services for the academic and scientific, professional and commercial business communities. Informa has some 150 offices in over 40 countries and employs approximately 8,000 staff around the world. Informa is the largest publicly-owned organiser of conferences and courses in the world with inThe Year The Year in Review 12 an output of around 8,000 events annually. Informa Financial Highlights 13 publishes over 2,100 subscription-based information Chairman’s Statement 14 services including academic journals, real-time news Chief Executive’s Review 16 Financial Review 19 R and structured databases of commercial intelligence. Board of Directors 24 eview Global Business Information Specialist Informa’s book business has more than 55,000 academic Advisers 26 Directors’ Report 27 and business titles. Corporate Governance Statement 38 Directors’ Remuneration Report 45 Corporate Responsibility 55 Financial Statements Financial Financial Statements 58 Independent Auditors’ Report – Group 59 Consolidated Income Statement 60 Consolidated Statement of Comprehensive Income 61 Consolidated Statement of Changes in Equity 62 Consolidated Statement of Financial Position 64 ”It was an extremely demanding year, Consolidated Cash Flow Statement 65 but one which -

Informa Corporate Structure
Corporate Structure From www.informa.com/Who-We-Are/Corporate-structure/ 5 March 2011 History Edward Lloyd pins the first ever copy of his shipping list to the wall of his coffee shop 1734 in Lombard Street, City of London. Richard Taylor publishes the first edition of The Philosophical Magazine, one of the 1798 first scientific journals produced by an independent company Dr William Francis joined Richard Taylor to found Taylor & Francis and continue the 1852 close links between the academic community and the company Taylor & Francis became a private limited company with leading scientists as 1936 directors and shareholders 1964 Investment & Property Studies founded as a part-time business International Business Communications Ltd (IBC) created to be an umbrella company 1971 for Investment & Property Studies and Legal Studies & Services The Institute for International Research (IIR) is launched by Irvine Laidlaw later to 1973 become Baron Laidlaw of Rothiemay – IIR becomes a specialist business conference organiser 1976 Euroforum established in Holland by IBC 1985 International Business Communications (Holdings) plc established Datamonitor is founded in London and produces its first ever report on the UK frozen 1989 food industry 1991 Launch of The Monaco Yacht Show by IIR Lloyd's List Publishing (LLP) and IBC Group plc merge to form the Informa Group 1998 plc Taylor & Francis successfully launched on the London Stock Exchange and shortly 1998 afterwards acquired the Routledge Group of companies Datamonitor, now a world-leading provider -

The Routledge Handbook of Neuroethics 1St Edition Pdf, Epub, Ebook
THE ROUTLEDGE HANDBOOK OF NEUROETHICS 1ST EDITION PDF, EPUB, EBOOK Syd Johnson | 9781138898295 | | | | | The Routledge Handbook of Neuroethics 1st edition PDF Book Toggle navigation Menu. Terms and Conditions. Inbunden Engelska, The volume spotlights new technologies and historical articulations of key problems, issues, and concepts and includes cross-referencing between chapters to highlight the complex interactions of concepts and ideas within neuroethics. Routledge has grown considerably as a result of organic growth and acquisitions of other publishing companies and other publishers' titles by its parent company. After decades of almost exclusive sociological focus, criminology has undergone a paradigm shift where the field is more interdisciplinary and this book combines perspectives from criminology and sociology with contributions from fields such as genetics, neuropsychology, and evolutionary psychology. The past two decades have seen unparalleled developments in our knowledge of the brain and mind. Abstract: Adding to a small but growing feminist literature, this article critically examines popular, contemporary American sex manuals from a feminist social constructionist perspective, focusing specifically on how these manuals construct gender and sexual norms. In , in the case Adams v. Inside Book Publishing. Asexualities and Media. Don't waste your time, continue to see developments from around the world through BOOK. Given its scope, the book will appeal to scientists and students interested in the debate on animal ethics, while also offering an important resource for future researchers. The Independent. Categories : Academic publishing companies Book publishing companies of the United Kingdom Companies based in Oxfordshire Reference publishers Vale of White Horse mergers and acquisitions establishments in England Publishing companies established in Journal of Sex and Marital Therapy. -

Informa Community Report:Layout 1
Informa and the Community Be involved ... Be Involved ...in Informa’s Community programme Earlier this year, we asked you to tell us what you thought about Informa’s current community strategy. An impressive 627 of you responded to our survey, representing divisions and companies as diverse as Datamonitor, IBI, ICBI, IIR, iMoneynet, Omega, Psychology Press, T&F and many more. So, what did you tell us? • 99% of those that replied to the survey knew about our annual, and global, Go Bananas event to raise money for WCRF • 86% of respondents would be interested in getting involved with a local charity to their own office • 58% would rather give time to community activities than to both Involvedvolunteering and fundraising I We asked you how you would like to support Informa’s community activities.Be This is what you told us... Give time (volunteering) 24% Neither Fundraise 6% 12% ... A bit of both 58% 2 [email protected] So, what should Informa’s community strategy look like? Developing Local Partnerships We’d like to support Informa businesses across the world in building partnerships with local charitable projects. In order to help make this happen, we are therefore going to devolve control over community funding to each local business unit. This means that local businesses units will now be responsible for dealing with requests for matched funding from staff. We have also consulted with local MDs who have agreed to give every Informa employee a day off to take part in a volunteering activity. Informa’s Matched Funding Guidelines • We’d like -

Top 10 Best-Selling Drugs of 2018 Fund US and EU Pharma R&D
Top 10 Best-Selling Drugs of 2018 Fund US and EU Pharma R&D Stephanie Yip Senior Analyst, Datamonitor Healthcare 2 / May 2019 © Informa UK Ltd 2019 (Unauthorized photocopying prohibited.) The top 10 most lucrative drugs in 2018 were worth approximately $87bn The top 10 commercially successful drugs each generated at least $6.5bn in 2018 The top 10 drugs with the highest global sales in continued to dominate the pharmaceutical market; 2018 each generated revenues of at least $6.5bn its global sales of $20.4bn were equivalent to (see Table 1). The total sales value of these drugs approximately 23% of the top 10 drugs’ total sales. amounted to an astounding $87bn in 2018. To put Easily head and shoulders above the rest, Humira’s this into context, this is comparable to the $90bn substantial lead margin of $10.7bn was worth more in pharmaceutical R&D expenditure across the US than the worldwide sales ($9.7bn) of the second and EU in 2016.1 Humira (adalimumab; AbbVie/Eisai) leading drug, Revlimid (lenalidomide; Celgene).2-5 Table 1. Highest-selling drugs in 2018 Rank Global sales, 2018 Brand (molecule) Companies (total sales by company) 1 $20.4bn Humira (adalimumab) AbbVie ($19,936m), Eisai (¥46,300m*) 2 $9.7bn Revlimid (lenalidomide) Celgene ($9,685m) 3 $7.6bn Opdivo (nivolumab) Bristol-Myers Squibb ($6,735m), Ono Pharmaceutical (¥92,400m*) 4 $7.5bn Enbrel (etanercept) Amgen ($5,014m), Pfi zer ($2,112m), Takeda (¥35,900*) 5 $7.2bn Herceptin (trastuzumab) Roche (CHF6,982m) 5 $7.2bn Keytruda (pembrolizumab) Merck & Co ($7,171m) 7 $7.1bn Avastin (bevacizumab) Roche (CHF6,849m) 8 $7.0bn Rituxan (rituximab) Roche (CHF6,752m) 9 $6.7bn Eylea (afl ibercept) Regeneron ($6,746m) 10 $6.6bn Xarelto (rivaroxaban) Bayer (€3,631m), Johnson & Johnson ($2,477m) *Sum of Q1–Q3 2018 sales and Q4 2017 sales. -

Contactless Payments 2006
Contactless Payments 2006 Reference Code: DMFS1839 Publication Date: 06/06 www.datamonitor.com Datamonitor USA Datamonitor Europe Datamonitor Germany Datamonitor Asia Pacific Datamonitor Japan 245 Fifth Avenue Charles House Kastor & Pollux Level 46 Wakamatsu Bldg 7F 4th Floor 108-110 Finchley Road Platz der Einheit 1 2 Park Street 3-3-6 Nihonbashi-Honcho New York, NY 10016 London NW3 5JJ 60327 Frankfurt Sydney NSW 2000 Chuo-ku USA United Kingdom Deutschland Australia Tokyo 103-0023 Japan t: +1 212 686 7400 t: +44 20 7675 7000 t: +49 69 9750 3119 t: +61 2 8705 6900 t: +813 6202 7681 f: +1 212 686 2626 f: +44 20 7675 7500 f: +49 69 9750 3320 f: +61 2 8705 6901 f: +813 5778 7537 e: [email protected] e: [email protected] e: [email protected] e: [email protected] e: [email protected] ABOUT DATAMONITOR Datamonitor plc is a premium business information company specializing in industry analysis. We help our clients, 5,000 of the world’s leading companies, to address complex strategic issues. Through our proprietary databases and wealth of expertise, we provide clients with unbiased expert analysis and in-depth forecasts for six industry sectors: Automotive, Consumer Markets, Energy, Financial Services, Healthcare, and Technology. Datamonitor maintains its headquarters in London and has regional offices in New York, Frankfurt, Sydney and Japan. FINANCIAL SERVICES CONSULTANCY We hope that the data and analysis in this report will help you to make informed and imaginative business decisions. However, it may be that the data or analysis does not perfectly meet your needs in which case Datamonitor is keen to help you further. -

Databases Available in the Research Information Center of the National Institute of Standards and Technology
United States Department of Commerce Technology Administration National Institute of Standards and Technology A111Q3 ACH3Z2 NIST Special Publication 842 Databases Available in the Research Information Center of the National Institute of Standards and Technology NIST PUBLICATIONS m^m.^ ^unningham AGRICOLA (National Agricultural Library) CANCERLINE (National Library of Medicine) CENDATA (Bureau of the Census) FEDERAL RESEARCH IN PROGRESS (NTIS) GPO MONTHLY CATALOG (Government Printing Office) MEDLINE (National Library of Medicine) NTIS (National Technical Information Service) NUCLEAR SCIENCE ABSTRACTS (DOE) OCCUPATIONAL SAFETY & HEALTH (NIOSH) SMOKING AND HEALTH (HHS) TRIS (Department of Transportation) 7he National Institute of Standards and Technology was established in 1988 by Congress to "assist industry in the development of technology . needed to improve product quality, to modernize manufacturing processes, to ensure product reliability . and to facilitate rapid commercialization ... of products based on new scientific discoveries." NIST, originally founded as the National Bureau of Standards in 1901, works to strengthen U.S. industry's competitiveness; advance science and engineering; and improve public health, safety, and the environment. One of the agency's basic functions is to develop, maintain, and retain custody of the national standards of measurement, and provide the means and methods for comparing standards used in science, engineering, manufacturing, commerce, industry, and education with the standards adopted or recognized by the Federal Government. As an agency of the U.S. Commerce Department's Technology Administration, NIST conducts basic and applied research in the physical sciences and engineering and performs related services. The Institute does generic and precompetitive work on new and advanced technologies. NIST's research facilities are located at Gaithersburg, MD 20899, and at Boulder, CO 80303. -

Information Specialist
COVER ARTWORK 08:Layout 1 25/3/08 14:32 Page 1 Global Information Specialist Mortimer House 37 – 41 Mortimer Street London, W1T 3JH T +44 (0)20 7017 5000 F +44 (0)20 7017 4286 www.informa.com Annual Report and Financial Statements 2007 COVER ARTWORK 08:Layout 1 25/3/08 14:32 Page 2 Informa Annual Report and Financial Statements 2007 What’s Inside Informa provides specialist, high value information to the global Academic & Scientific, Professional, 03 Chairman’s and Chief Executive’s Statement and Commercial markets via Publishing, Events 06 Financial Highlights and Performance Improvement. 08 Chairman’s and At the heart of every Informa product and service is research-based, Chief Executive’s Report proprietary information for a highly targeted expert audience. Informa Business Streams publishes approximately 2,500 subscription based products and services 12 Publishing delivered both electronically and in hardcopy, and 45,000 books. Each year 16 Performance Improvement (PI) Informa produces over 12,000 events around the world powered by a 20 Events marketing database of over 20 million contacts. We have an extensive portfolio Divisions of prominent brands including Lloyd’s List, Routledge, Taylor & Francis, IIR, IBC, 24 Academic & Scientific AchieveGlobal, ESI, Euroforum and Datamonitor. Informa operates in over 80 28 Professional countries, employing more than 10,000 people. 32 Commercial 36 Trading Outlook 40 Financial Review 45 Officers and Advisers 48 Corporate and Risk Information 54 Senior Independent Director’s Report 60 Directors’ Remuneration Report Financial Statements Publishing Performance Events 69 Statement of Directors’ Revenue £495.0m Improvement Revenue £408.8m Responsibilities Pages 12 - 15. -

Datamonitor Plc Informa Acquisitions Limited Greenhill & Co
THIS DOCUMENT IS IMPORTANT AND REQUIRES YOUR IMMEDIATE ATTENTION. If you are in any doubt as to what action you should take, you are recommended to seek your own personal financial advice immediately from your stockbroker, bank manager, solicitor, accountant or other independent financial adviser authorised under the Financial Services and Markets Act 2000 if you are resident in the United Kingdom or, if not, from another appropriately authorised independent financial adviser. If you have sold or otherwise transferred all of your Datamonitor Shares (other than pursuant to the Offer), please send this document and the reply-paid envelope (but not the personalised Form of Acceptance) as soon as possible to the purchaser or transferee, or to the stockbroker, bank or other agent through whom the sale or transfer was effected, for onward transmission to the purchaser or transferee. However, these documents must not be forwarded, distributed or transmitted in, into or from any jurisdiction where to do so would violate the laws in that jurisdiction. If you have sold or otherwise transferred only part of your holding of Datamonitor Shares you should retain these documents. Greenhill & Co. International LLP, which is authorised and regulated by the FSA, is acting exclusively for Informa and Informa Acquisitions and no one else in connection with the Offer and will not be responsible to anyone other than Informa and Informa Acquisitions for providing the protections afforded to clients of Greenhill & Co. International LLP nor for providing advice in connection with the Offer or any other matters referred to herein. LongAcre Partners Limited, which is authorised and regulated by the FSA, is acting exclusively for Datamonitor and no one else in connection with the Offer and will not be responsible to anyone other than Datamonitor for providing the protections afforded to clients of LongAcre Partners Limited or for providing advice in connection with the Offer or any other matters referred to herein.