Volume 37 Number 07
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Expect the Unexpected: Building Business Value in a Changing World
Expect the Unexpected: Building business value in a changing world kpmg.com KPMG INTERNATIONAL PART 1 b | Expect the Unexpected: Building business value in a changing world In this report we offer a starting point for discussion. We present a system of ten sustainability megaforces that will impact each and every business over the next 20 years. We want to build awareness that these forces do not act alone in predictable ways. They are interconnected. They interact. Disclaimer: Throughout this document, "KPMG" ["we," "our," and "us"] refers to KPMG International, a Swiss entity that serves as a coordinating entity for a network of independent member firms operating under the KPMG name, KPMG's Climate Change and Sustainability practice, and/or to any one or more of such firms. KPMG International provides no client services. © 2012 KPMG International Cooperative (“KPMG International”), a Swiss entity. Member firms of the KPMG network of independent firms are affiliated with KPMG International. KPMG International provides no client services. All rights reserved. Expect the Unexpected: Building business value in a changing world | c Foreword usinesses today are operating in an ever more At KPMG’s network of firms we have always been at the interconnected and globalized world. Supply chains forefront of developments that shape business behavior. Bstretch across continents and are vulnerable to We are working with organizations to help them understand disruption. Consumer demands and government policies the forces at work that will influence markets and impact are changing rapidly and will impact your bottom line if your profitability in the medium to long term. -

Novartis Swaps Two Avexis Executives for One Following
No. 3969 August 23, 2019 and CSO that the Kaspars have not been involved in the Novartis subsidiary’s op- erations since early May. Asked whether more AveXis employees will exit the company due to the preclini- cal safety data manipulation revealed on 6 August or whether other actions will be taken by Novartis in relation to the matter, Althoff told Scrip: “We cannot comment on the specifics of who is involved, but we can confirm this issue was not widespread, ei- ther in our overall dataset nor in our com- pany or any function within our company and was limited to a few individuals.” Novartis CEO Vas Narasimhan said in a 7 August conference call about the Zol- gensma data controversy that the compa- ny was in the process of “exiting the small number of AveXis scientists involved” in the data manipulation, which occurred Novartis Swaps Two AveXis before Novartis bought the gene therapy firm. (Also see “Novartis CEO Explains De- lay In Telling US FDA About Zolgensma Executives For One Following Data Fraud: We Wanted To Understand It First” - Pink Sheet, 7 Aug, 2019.) Zolgensma Data Manipulation Novartis acquired AveXis for $8.7bn MANDY JACKSON [email protected] last year. (Also see “Novartis Goes Big On Gene Therapy With $8.7bn AveXis Acquisi- ovartis AG said when it disclosed the Zolgensma BLA was manipulated. Page tion” - Scrip, 9 Apr, 2018.) Zolgensma was that preclinical safety data for Zol- Bouchard, whose most recent function was the lead asset in AveXis’s R&D pipeline and Ngensma were manipulated in the bi- global head of preclinical safety for Novar- won US Food and Drug Administration ologic license application for the gene ther- tis Institutes of Biomedical Research (NIBR), approval in May, becoming the world’s apy that the company would be “exiting” was named senior vice president and chief most expensive drug with a $2m list price. -

Andrew Goldstein, Ph.D., Is an Assistant Professor Of
Andrew S. Goldstein, Ph.D. Biography: Andrew Goldstein, Ph.D., is an Assistant Professor of Molecular, Cell and Developmental Biology and Assistant Professor of Urology in the David Geffen School of Medicine at the University of California, Los Angeles. Dr. Goldstein is a member of the Broad Stem Cell Research Center and the Jonsson Comprehensive Cancer Center at UCLA. Dr. Goldstein majored in Biochemistry and Molecular Biology at Dartmouth College before receiving his Ph.D. in Molecular Biology under the mentorship of Dr. Owen Witte at UCLA. He went on to start his own research group as a Fellow of the Broad Stem Cell Research Center at UCLA in 2011 before joining the faculty as an Assistant Professor in 2016. Dr. Goldstein’s research has been supported by a Prostate Cancer Foundation Young Investigator Award, Department of Defense PCRP Idea Development Award and an American Cancer Society Research Scholar Grant, and his work is currently supported by an NIH/NCI R01. He has been awarded the 2018 American Cancer Society Giants of Science Hope Award, 2019 SBUR Young Investigator Award and 2019 Rose Hills Innovator Award. At UCLA, Dr. Goldstein serves on committees for the Jonsson Comprehensive Cancer Center, the SPORE in Prostate Cancer, and the graduate program in Cell and Developmental Biology. He joined the Society for Basic Urologic Research in 2019 and currently participates as part of the membership committee (2020-present). Research Interests: Research in Dr. Goldstein’s laboratory is focused on defining factors that regulate prostate progenitor cell aging and transformation with an emerging interest in prostate epithelial metabolism. -

UNIVERSITY of CALIFORNIA Los Angeles Gene Editing of Bruton's
UNIVERSITY OF CALIFORNIA Los Angeles Gene Editing of Bruton’s Tyrosine Kinase for Treatment of X-Linked Agammaglobulinemia A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Molecular Biology by David Gray 2020 © Copyright by David Gray 2020 ABSTRACT OF THE DISSERTATION Gene Editing of Bruton’s Tyrosine Kinase for Treatment of X-Linked Agammaglobulinemia by David Gray Doctor of Philosophy in Molecular Biology University of California, Los Angeles, 2020 Professor Donald Barry Kohn, Chair X-Linked Agammaglobulinemia (XLA) is a primary immunodeficiency characterized by a lack of mature B lymphocytes and antibody production. Patients with XLA have loss of function mutations in the Bruton’s Tyrosine Kinase (BTK) gene. The standard of care for XLA is immunoglobulin supplementation, which has a profound effect on patient wellbeing and life expectancies. However, immunoglobulin supplementation requires frequent, expensive injections throughout a patient’s life and patients remain susceptible to certain recurring illnesses. The only permanent cure for XLA is an allogeneic hematopoietic stem cell (HSC) transplant, though it is rarely performed due to the associated risks. Gene therapy-based methods to replace or repair the BTK gene in autologous HSCs provide an alternative with the benefits of a permanent cure for XLA while circumventing much of the risk. While previous efforts to deliver a functional copy of the BTK gene using viral vector mediated gene transfer have shown promise, these vectors carry a risk of insertional oncogenesis ii which may not be tolerated for treatment of XLA. Instead, this dissertation lays a foundation for targeted integration of a functional copy of the BTK gene into HSCs using Cas9 endonuclease mediated gene editing to drastically reduce those risks. -
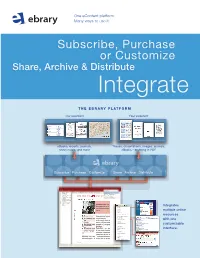
The EBRARY Platform
One eContent platform. Many ways to use it. Subscribe, Purchase or Customize Share, Archive & Distribute Integrate THE EBRARY PLatFORM Our eContent Your eContent eBooks, reports, journals, Theses, dissertations, images, journals, sheet music, and more eBooks – anything in PDF Subscribe Purchase Customize Share Archive Distribute SULAIR Select Collections SUL EBRARY COLLECTIONS Select Collections TABLE OF CONTENTS Infotools All ebrary Collections CONTRIBUTORS Byron Hoyt Sheet Music (Browse) INTRODUCTION Immigration Commision Reports (Dillingham) Contents BUSINESS: A USER’S GUIDE Women and Child Wage Earners in the U.S. CSLI Linguistics and Philosophy BEST PRACTICE SUL Books in the Public Domain MANAGEMENT CHECKLISTS Medieval and Modern Thought Text Project ACTIONLISTS MANAGEMENT LIBRARY BUSINESS THINKERS AND MANA DICTIONARY WORLD BUSINESS ALMANAC Highlights Notes BUSINESS INFORMATION SOURC INDEX CREDITS InfoTools Integrates Define Explain Locate multiple online Translate Search Document... resources Search All Documents... Search Web Search Catalog with one Highlight Add to Bookshelf customizable Copy Text... Copy Bookmark interface. Print... Print Again Toggle Automenu Preferences... Help About ebrary Reader... EASY TO USE. Subscribe, Purchase AFFORDABLE. or Customize your ALW ay S AVAILABLE. eBook selection NO CHECK-OUTS. Un I Q U E S U B SC R I B E T O G R OWI ng E B O O K D ataba S E S W I T H SIMULtanEOUS, MULTI-USER ACCESS. RESEARCH TOOLS. ACADEMIC DATABASES Academic Complete includes all academic databases listed below. FREE MARCS. # of # of Subject Titles* Subject Titles* Business & Economics 6,300 Language, Literature 3,400 & Linguistics “ebrary’s content is Computers & IT 2,800 Law, International Relations 3,800 multidisciplinary and Education 2,300 & Public Policy Engineering & Technology 3,700 supports our expanding Life Sciences 2,000 (includes Biotechnolgy, History & Political Science 4,800 Agriculture, and (also includes a bonus selection faculty and curriculum. -

Annual Copyright License for Corporations
Responsive Rights - Annual Copyright License for Corporations Publisher list Specific terms may apply to some publishers or works. Please refer to the Annual Copyright License search page at http://www.copyright.com/ to verify publisher participation and title coverage, as well as any applicable terms. Participating publishers include: Advance Central Services Advanstar Communications Inc. 1105 Media, Inc. Advantage Business Media 5m Publishing Advertising Educational Foundation A. M. Best Company AEGIS Publications LLC AACC International Aerospace Medical Associates AACE-Assn. for the Advancement of Computing in Education AFCHRON (African American Chronicles) AACECORP, Inc. African Studies Association AAIDD Agate Publishing Aavia Publishing, LLC Agence-France Presse ABC-CLIO Agricultural History Society ABDO Publishing Group AHC Media LLC Abingdon Press Ahmed Owolabi Adesanya Academy of General Dentistry Aidan Meehan Academy of Management (NY) Airforce Historical Foundation Academy Of Political Science Akademiai Kiado Access Intelligence Alan Guttmacher Institute ACM (Association for Computing Machinery) Albany Law Review of Albany Law School Acta Ecologica Sinica Alcohol Research Documentation, Inc. Acta Oceanologica Sinica Algora Publishing ACTA Publications Allerton Press Inc. Acta Scientiae Circumstantiae Allied Academies Inc. ACU Press Allied Media LLC Adenine Press Allured Business Media Adis Data Information Bv Allworth Press / Skyhorse Publishing Inc Administrative Science Quarterly AlphaMed Press 9/8/2015 AltaMira Press American -

Global Business Information Specialist Informa’S Book Business Has More Than 55,000 Academic Advisers 26 Directors’ Report 27 and Business Titles
Business Profile Business WHO WE ARE Business Profile 02 Annual Report & Financial Statements 2009 Our Business 03 Informa at a Glance 04 Academic Information 06 Professional & Commercial Information 08 Events & Training 10 Informa plc is a leading international provider of specialist information and services for the academic and scientific, professional and commercial business communities. Informa has some 150 offices in over 40 countries and employs approximately 8,000 staff around the world. Informa is the largest publicly-owned organiser of conferences and courses in the world with inThe Year The Year in Review 12 an output of around 8,000 events annually. Informa Financial Highlights 13 publishes over 2,100 subscription-based information Chairman’s Statement 14 services including academic journals, real-time news Chief Executive’s Review 16 Financial Review 19 R and structured databases of commercial intelligence. Board of Directors 24 eview Global Business Information Specialist Informa’s book business has more than 55,000 academic Advisers 26 Directors’ Report 27 and business titles. Corporate Governance Statement 38 Directors’ Remuneration Report 45 Corporate Responsibility 55 Financial Statements Financial Financial Statements 58 Independent Auditors’ Report – Group 59 Consolidated Income Statement 60 Consolidated Statement of Comprehensive Income 61 Consolidated Statement of Changes in Equity 62 Consolidated Statement of Financial Position 64 ”It was an extremely demanding year, Consolidated Cash Flow Statement 65 but one which -

Informa Corporate Structure
Corporate Structure From www.informa.com/Who-We-Are/Corporate-structure/ 5 March 2011 History Edward Lloyd pins the first ever copy of his shipping list to the wall of his coffee shop 1734 in Lombard Street, City of London. Richard Taylor publishes the first edition of The Philosophical Magazine, one of the 1798 first scientific journals produced by an independent company Dr William Francis joined Richard Taylor to found Taylor & Francis and continue the 1852 close links between the academic community and the company Taylor & Francis became a private limited company with leading scientists as 1936 directors and shareholders 1964 Investment & Property Studies founded as a part-time business International Business Communications Ltd (IBC) created to be an umbrella company 1971 for Investment & Property Studies and Legal Studies & Services The Institute for International Research (IIR) is launched by Irvine Laidlaw later to 1973 become Baron Laidlaw of Rothiemay – IIR becomes a specialist business conference organiser 1976 Euroforum established in Holland by IBC 1985 International Business Communications (Holdings) plc established Datamonitor is founded in London and produces its first ever report on the UK frozen 1989 food industry 1991 Launch of The Monaco Yacht Show by IIR Lloyd's List Publishing (LLP) and IBC Group plc merge to form the Informa Group 1998 plc Taylor & Francis successfully launched on the London Stock Exchange and shortly 1998 afterwards acquired the Routledge Group of companies Datamonitor, now a world-leading provider -

UCLA Electronic Theses and Dissertations
UCLA UCLA Electronic Theses and Dissertations Title Non-mutated kinases in prostate cancer metastasis: drivers and therapeutic targets Permalink https://escholarship.org/uc/item/7615v8xd Author Faltermeier, Claire Publication Date 2016 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles Non-mutated kinases in metastatic prostate cancer: drivers and therapeutic targets A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Molecular Biology by Claire Faltermeier 2016 © Copyright by Claire Faltermeier 2016 ABSTRACT OF THE DISSERTATION Non-mutated kinases in prostate cancer: drivers and therapeutic targets by Claire Faltermeier Doctor of Philosophy in Molecular Biology University of California, Los Angeles, 2016 Professor Hanna K. A. Mikkola, Chair Metastatic prostate cancer lacks effective treatments and is a major cause of death in the United States. Targeting mutationally activated protein kinases has improved patient survival in numerous cancers. However, genetic alterations resulting in constitutive kinase activity are rare in metastatic prostate cancer. Evidence suggests that non-mutated, wild-type kinases are involved in advanced prostate cancer, but it remains unknown whether kinases contribute mechanistically to metastasis and should be pursued as therapeutic targets. Using a mass- spectrometry based phosphoproteomics approach, we identified tyrosine, serine, and threonine kinases -

Inner Workings: Tyrosine Kinases, Their Discovery and Impact
INNER WORKINGS Inner Workings: Tyrosine kinases, their discovery and impact Jessica Marshall Science Writer At the same time, Witte and his colleagues reported that the cancer-causing Abelson virus, which acts in mice, worked via another protein that added a phosphorous to tyro- While working in his laboratory at the Salk as a protein kinase: an enzyme that adds sine. But, this occurred through self-phos- Institute in 1979, Tony Hunter took a a phosphate to another protein (2). The phorylation (4). shortcut. Hunter decided not to make up buffer’s pH had fallen slightly as it sat on Tyrosine kinases act as a signal relay that freshbufferforhiselectrophoresisrun.That the bench, which caused two amino acids— control many cellular pathways, including cell decision would alter his career, significantly phosphothreonine and phosphotyrosine—to growth. When the kinases become mutated influence the field of cancer biology, and separate from each other on the electropho- in cancer, cell division spirals out of control. ultimately lead to new cancer treatments. resis plate, when normally they would have This understanding ultimately led to the Hunter was studying Rous sarcoma virus, run together. This separation revealed that development of a whole new class of cancer the first known cancer-causing virus, reported the target for the cancer-causing protein drugs known as tyrosine kinase inhibitors. in 1911 by Peyton Rous, who showed it kinase was tyrosine. “People immediately realized that many caused cancer in chickens. Rous’sdiscovery, It was the first report of a tyrosine kinase kinases may be tyrosine kinases,” says Stanley which earned him the Nobel Prize in 1966, (3), a class of proteins that would prove to be Lipkowitz of the National Cancer Institute. -

Profile of Charles L. Sawyers
PROFILE Profile of Charles L. Sawyers harles Sawyers is a rock star in his Ras, a handful of research teams across own right. Last year, Sawyers, the country were hot on the trail of other Cchair of the Human Oncology oncogenes. Witte focused on the Phila- and Pathogenesis Program at delphia Chromosome, a genetic aberra- Memorial Sloan–Kettering Cancer Center tion that juxtaposes two genes, dubbed and a member of the National Academy BCR and ABL, when human chromo- of Sciences, posed with singer Debbie somes 9 and 22 swap segments, producing Harry of the rock band Blondie to pro- a hybrid enzyme in blood cells that jams mote cancer research in a campaign the cells’ growth restraints and leads to sponsored by the Geoffrey Beene Cancer CML. Patients with CML have a pro- Research Center. The unlikely assemblage fusion of frenetically dividing white blood of rock star and researcher shined the cells. Left unchecked, the disease can kill spotlight on pioneering efforts in trans- within 3 years of diagnosis. “This was an lational cancer research. Few physicians example of a cancer where the genetic deserve that spotlight more than Sawyers, abnormality was clear. That’s what at- who co-discovered the targeted cancer tracted me to Owen’s lab,” says Sawyers, drug, Gleevec, forging a path to cancer who joined the laboratory as a post- treatment that has now become increas- doctoral fellow in 1989. That move was ingly common. merely the opening act to a career punc- Born to physicians who served as a tuated by startling revelations, fruitful source of inspiration, Sawyers grew up in partnerships, life-saving discoveries, and a Nashville, Tennessee household where even heart-wrenching disappointments. -

Profile of Charles L. Sawyers
PROFILE Profile of Charles L. Sawyers harles Sawyers is a rock star in his Ras, a handful of research teams across own right. Last year, Sawyers, the country were hot on the trail of other Cchair of the Human Oncology oncogenes. Witte focused on the Phila- and Pathogenesis Program at delphia Chromosome, a genetic aberra- Memorial Sloan–Kettering Cancer Center tion that juxtaposes two genes, dubbed and a member of the National Academy BCR and ABL, when human chromo- of Sciences, posed with singer Debbie somes 9 and 22 swap segments, producing Harry of the rock band Blondie to pro- a hybrid enzyme in blood cells that jams mote cancer research in a campaign the cells’ growth restraints and leads to sponsored by the Geoffrey Beene Cancer CML. Patients with CML have a pro- Research Center. The unlikely assemblage fusion of frenetically dividing white blood of rock star and researcher shined the cells. Left unchecked, the disease can kill spotlight on pioneering efforts in trans- within 3 years of diagnosis. “This was an lational cancer research. Few physicians example of a cancer where the genetic deserve that spotlight more than Sawyers, abnormality was clear. That’s what at- who co-discovered the targeted cancer tracted me to Owen’s lab,” says Sawyers, drug, Gleevec, forging a path to cancer who joined the laboratory as a post- treatment that has now become increas- doctoral fellow in 1989. That move was ingly common. merely the opening act to a career punc- Born to physicians who served as a tuated by startling revelations, fruitful source of inspiration, Sawyers grew up in partnerships, life-saving discoveries, and a Nashville, Tennessee household where even heart-wrenching disappointments.