The Interstellar Medium Worksheet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Messengers from Outer Space
The complete absence of national laboratories for standardizing radiation measurements and the lack of physics departments in most radiotherapy centres in Latin America warrant the setting up of one or more regional dosimetry facilities, the functions of which would be primarily to calibrate dosimeters; to provide local technical assistance by means of trained staff; to check radiation equipment and dosimeters; to arrange a dose-intercomparison service; and to co-operate with local personnel dosimetry services. To be most effective, this activity should be in the charge of local personnel, with some initial assistance in the form of equipment and experts provided by the Agency. Although the recommendations were presented to the IAEA as the or ganization responsible for the setting up of the panel, the participants re commended that the co-operation of the World Health Organization and the Pan-American Health Organization should be invited. It was also suggested that the panel report be circulated to public health authorities in the countries represented. MESSENGERS FROM OUTER SPACE Although no evidence has yet been confirmed of living beings reaching the earth from space, it has been estimated that hundreds of tons of solid matter arrive every day in the form of meteorites or cosmic dust particles. Much is destroyed by heat in the atmosphere but fragments recovered can give valuable information about what has been happening in the universe for billions of years. Some of the results of worldwide research on meteorites were given at a symposium in Vienna during August. During six days of discussions a total of 73 scientific papers was present ed and a special meeting was held for the purpose of improving international co-operation in the research. -
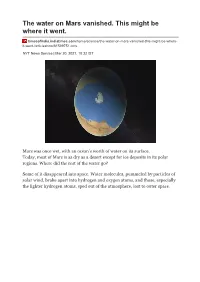
The Water on Mars Vanished. This Might Be Where It Went
The water on Mars vanished. This might be where it went. timesofindia.indiatimes.com/home/science/the-water-on-mars-vanished-this-might-be-where- it-went-/articleshow/81599751.cms NYT News Service | Mar 20, 2021, 10:32 IST Mars was once wet, with an ocean’s worth of water on its surface. Today, most of Mars is as dry as a desert except for ice deposits in its polar regions. Where did the rest of the water go? Some of it disappeared into space. Water molecules, pummeled by particles of solar wind, broke apart into hydrogen and oxygen atoms, and those, especially the lighter hydrogen atoms, sped out of the atmosphere, lost to outer space. A tall outcropping of rock, with layered deposits of sediments in the distance, marking a remnant of an ancient, long-vanished river delta in Jezero Crater, are pictured in this undated image taken by NASA's Mars rover Perseverance. (Reuters) But most of the water, a new study concludes, went down, sucked into the red planet’s rocks. And there it remains, trapped within minerals and salts. Indeed, as much as 99% of the water that once flowed on Mars could still be there, the researchers estimated in a paper published this week in the journal Science. Data from the past two decades of robotic missions to Mars, including NASA ’s Curiosity rover and the Mars Reconnaissance Orbiter, showed a wide distribution of what geologists call hydrated minerals. “It became very, very clear that it was common and not rare to find evidence of water alteration,” said Bethany Ehlmann, a professor of planetary science at the California Institute of Technology and one of the authors of the paper. -

The Protection of Frequencies for Radio Astronomy 1
JOURNAL OF RESEARCH of the National Bureau of Standards-D. Radio Propagation Vol. 67D, No. 2, March- April 1963 b The Protection of Frequencies for Radio Astronomy 1 R. 1. Smith-Rose President, International Scientific Radio Union (R eceived November 5, 1962) The International T elecommunications Union in its Geneva, 1959 R adio R egulations r recognises the Radio Astronomy Service in t he two following definitions: N o. 74 Radio A st1" onomy: Astronomy based on t he reception of waves of cos mi c origin. No. 75 R adio A st1"onomy Se1"vice: A service involving the use of radio astronomy. This service differs, however, from other r adio services in two important respects. 1. It does not itself originate any radio waves, and therefore causes no interference to any other service. L 2. A large proportion of its activity is conducted by the use of reception techniques which are several orders of magnit ude )]).ore sensitive than those used in other ra dio services. In order to pursue his scientific r esearch successfully, t he radio astronomer seeks pro tection from interference first, in a number of bands of frequencies distributed t hroughout I t he s p ~ct run:; and secondly:. 1~10r e complete and s p ec i~c prote.ction fOl: t he exact frequency bands III whIch natural radIatIOn from, or absorptIOn lD, cosmIc gases IS known or expected to occur. The International R egulations referred to above give an exclusive all ocation to one freq uency band only- the emission line of h ydrogen at 1400 to 1427 Mc/s. -

What Are Cosmic Rays?!
WhatWWhatWhhaatt areaarearree CosmicCCosmicCoossmmiicc Rays?!RRays?!Raayyss??!! By Hayanon Translated by Y. Noda and Y. Kamide Supervised by Y. Muraki ᵶᵶᵋᵰᵿᶗᶑᵘᴾᴾᵱᶇᶀᶊᶇᶌᶅᶑᴾᶍᶄᴾᵡᶍᶑᶋᶇᶁᴾᵰᵿᶗᶑᵋᵰᵿᶗᶑᵘᵘᴾᴾᵱᶇᶀᶊᶊᶇᶌᶅᶑᴾᶍᶄᴾᵡᶍᶑᶋᶇᶁᴾᵰᵿᶗᶑ Have you ever had an X-ray examination Scientists identified three types at the hospital? In 1896, a German of radiation: positively-charged alpha physicist, W. C. Röntgen, astonished people particles, negatively-charged beta particles, with an image of bones captured through and uncharged gamma rays. In 1903, M. the use of X-rays. He had just discovered Curie along with her husband, P. Curie, and the new type of rays emitted by a discharge Becquerel, won the Nobel prize in physics. device. He named them X-rays. Because Furthermore, M. Curie was awarded the of their high penetration ability, they are Nobel prize in chemistry in 1911. able to pass through flesh. Soon after, it Certain types of radiation including was found that excessive use of X-rays can X-rays are now used for many medical cause harm to bodies. purposes including examining inside the In that same year, a French scientist, body, treating cancer, and more. Radiation, A. H. Becquerel, found that a uranium however, could be harmful unless the compound also gave off mysterious rays. amount of radiation exposure is strictly To his surprise, they could penetrate controlled. wrapping paper and expose a photographic The work with radium by M. Curie later film generating an image of the uranium led to the breakthrough discovery of compound. The uranium rays had similar the radiation coming from space. These characteristics as those of X-rays, but were cosmic rays were discovered by an Austrian determined to be different from them. -

Problems with Panspermia Or Extraterrestrial Origin of Life Scenarios As Found on the IDEA Center Website At
Problems with Panspermia or Extraterrestrial Origin of Life Scenarios As found on the IDEA Center website at http://www.ideacenter.org Panspermia is the theory that microorganisms or biochemical compounds from outer space are responsible for originating life on Earth and possibly in other parts of the universe where suitable atmospheric conditions exist. Essentially, it is a hypothesis which states that life on earth came from outer space. It has been popularized in countless science fiction works, however some scientists, including the discoverer of the structure of DNA, Francis Crick, have advocated panspermia. There are generally about 3 different "flavors" of panspermia: 1. Naturalistic Panspermia where life evolves on another planet, and naturally gets ejected off the planet and come to rest on earth. 2. Directed Panspermia where intelligent life on other planets intentionally seeded other planets with their own form of life. 3. Intelligent Design Panspermia, where intelligent beings from another planet came to earth and designed life here. Each type of panspermia will be briefly discussed below: 1. Naturalistic Panspermia where life evolves on another planet, and naturally gets ejected off the planet and come to rest on earth. Naturalistic panspermia has gained popularity because some have recognized that life on earth appears very soon after the origin of conditions which would allow it to exist. Many scientists believed that if life had originated on earth it would have taken many hundreds of millions, or even billions of years to do so. Life appears perhaps less than 200 million after the origin of conditions at which life could exist. -

Owning Outer Space Ezra J
Northwestern Journal of International Law & Business Volume 20 Issue 1 Fall Fall 1999 Owning Outer Space Ezra J. Reinstein Follow this and additional works at: http://scholarlycommons.law.northwestern.edu/njilb Part of the Air and Space Law Commons Recommended Citation Ezra J. Reinstein, Owning Outer Space, 20 Nw. J. Int'l L. & Bus. 59 (1999-2000) This Article is brought to you for free and open access by Northwestern University School of Law Scholarly Commons. It has been accepted for inclusion in Northwestern Journal of International Law & Business by an authorized administrator of Northwestern University School of Law Scholarly Commons. Owning Outer Space Ezra J. Reinstein * I. INTRODUCTION What do we want from space? We want the knowledge we can gain from scientific research; we can learn much about the Earth and its inhabitants, as well as the universe around us, by studying space. We want to explore, to satisfy the thirst for adventure and conquest imagined in countless science fiction books and films. We want to improve our collective lot down here on Earth. Space offers the potential for practically limitless wealth, some already being exploited, some we may only harness in the distant future, and un- doubtedly some we cannot begin to guess. Already the wealth of space is being developed in the form of telecommunications and remote satellite ob- servation. The private-sector investment in telecommunications satellites alone was projected to total $54.3 billion (including launch) between 1996 and 20001 -- and this figure doesn't include other commercial space ven- tures, nor does it include investment in Russian and Chinese satellites. -

Crater: How Cosmic Rays Affect Humans
Lunar Reconnaissance Orbiter: (CRaTER) Audience CRaTER: Grades 6-8 How Cosmic Rays Affect Time Recommended 30-45 minutes Humans AAAS STANDARDS • 1B/1: Scientific investigations usually involve the col- lection of relevant evidence, the use of logical reasoning, Learning Objectives: and the application of imagination in devising hypoth- • Students will be able to describe why cosmic rays are dangerous to eses and explanations to make sense of the collected evidence. astronauts. • Students will learn to design a scientific instrument. • 3A/M2: Technology is essential to science for such purposes as access to outer space and other remote • Students will think critically about how to protect astronauts from cosmic locations, sample collection and treatment, measurement, rays. data collection and storage, computation, and communi- cation of information. NSES STANDARDS Preparation: Content Standard A (5-8): Abilities necessary to do scien- None tific inquiry: c. Use appropriate tools to gather, analyze and inter- pret data. Background Information: d. Develop descriptions and explanations using On their trips to and from the Moon, Apollo astronauts saw small white flashes evidence. of light while in the dark—even with their eyes closed. They usually saw no e. Think critically and logically to make relationships more than a couple each minute, although at least one astronaut saw so many between evidence and explanations he had trouble sleeping. What caused these flashes? Content Standard E (5-8): Science and Technology: b. Technology is essential to science, because it The answer is cosmic rays. Because of their high speeds, thousands of these provides instruments and techniques that enable observations of objects and phenomena that cosmic rays were passing through the bodies of the Apollo astronauts every are otherwise unobservable due to factors such second. -

Astrobiology Life in the Universe
Astrobiology Astrobiology is the study of the origin, evolution, distribution, and future of life in the universe. In simplest terms, it is the study of life in the universe–both on Earth and off it. It combines the search for habitable environments in the Solar System and beyond with research into the evolution and adaptability of life here on Earth. By knitting together research in astrophysics, earth science, and heliophysics as well as planetary science, astrobiology seeks to answer fundamental scientific questions about life: how it begins and evolves; what biological, planetary, and cosmic conditions must exist in order for it to take hold; and whether there is/was/can be life elsewhere in the galaxy. Dr. Alka Misra Assistant Professor Department of Mathematics & Astronomy University of Lucknow What is Astrobiology! Astrobiology is the study of life in the Universe – where it is, how it came to be there, what it is like, and where it might be going. As the only life we know about for sure is on Earth, a lot of astrobiology is about trying to predict where we might find life elsewhere. Astrobiology is the study of the origin, evolution, distribution, and future of life in the universe. This interdisciplinary field encompasses the search for habitable environments in our Solar System and habitable planets outside our Solar System, the search for evidence of prebiotic chemistry, laboratory and field research into the origins and early evolution of life on Earth, and studies of the potential for life to adapt to challenges on Earth and in outer space. -

Space Based Astronomy Educator Guide
* Space Based Atronomy.b/w 2/28/01 8:53 AM Page C1 Educational Product National Aeronautics Educators Grades 5–8 and Space Administration EG-2001-01-122-HQ Space-Based ANAstronomy EDUCATOR GUIDE WITH ACTIVITIES FOR SCIENCE, MATHEMATICS, AND TECHNOLOGY EDUCATION * Space Based Atronomy.b/w 2/28/01 8:54 AM Page C2 Space-Based Astronomy—An Educator Guide with Activities for Science, Mathematics, and Technology Education is available in electronic format through NASA Spacelink—one of the Agency’s electronic resources specifically developed for use by the educa- tional community. The system may be accessed at the following address: http://spacelink.nasa.gov * Space Based Atronomy.b/w 2/28/01 8:54 AM Page i Space-Based ANAstronomy EDUCATOR GUIDE WITH ACTIVITIES FOR SCIENCE, MATHEMATICS, AND TECHNOLOGY EDUCATION NATIONAL AERONAUTICS AND SPACE ADMINISTRATION | OFFICE OF HUMAN RESOURCES AND EDUCATION | EDUCATION DIVISION | OFFICE OF SPACE SCIENCE This publication is in the Public Domain and is not protected by copyright. Permission is not required for duplication. EG-2001-01-122-HQ * Space Based Atronomy.b/w 2/28/01 8:54 AM Page ii About the Cover Images 1. 2. 3. 4. 5. 6. 1. EIT 304Å image captures a sweeping prominence—huge clouds of relatively cool dense plasma suspended in the Sun’s hot, thin corona. At times, they can erupt, escaping the Sun’s atmosphere. Emission in this spectral line shows the upper chro- mosphere at a temperature of about 60,000 degrees K. Source/Credits: Solar & Heliospheric Observatory (SOHO). SOHO is a project of international cooperation between ESA and NASA. -

Panspermia — the Theory That Life Came from Outer Space
CEN Tech. J., vol. 7(1), 1993, pp. 82–87 Panspermia — The Theory that Life came from Outer Space DR JERRY BERGMAN ABSTRACT In debates about the creation-evolution controversy, many evolutionists allege that the existing naturalistic origin of life theory is generally regarded as highly possible. That these theories are in fact not tenable is shown by the fact that many prominent evolutionists now hypothesize an alternate method. Since the theistic world view is unacceptable, they thus assume a set of conditions elsewhere in the solar system or the universe which are theorized to be more favourable for the origin of life. The recognition that the conditions on the earth are such that the origin of life is close to impossible forces this approach if a naturalistic world view is maintained. Nowhere does the literature reveal as vividly the impossibility of a naturalistic origin of life on the earth than in this area. The fact that an entirely hypothetical scenario has been proposed which is supported by virtually no evidence and only, at best, indirect inferences which can be interpreted as evidence, forces a review of the theory of panspermia. Much science fiction has in time become science fact — journeying to the moon, for example —and this should not surprise us. Many science fiction writers are scientists by profession, or at least trained in science at the graduate level. Isaac Asimov, a Ph.D. in biochemistry, is the best known example. Writing good science fiction requires both a good grasp of science fact and a vivid imagination. One of the latest science fictions which is now becoming ‘respectable’ science theory is panspermia, the belief that the source of life on earth was from other worlds. -

Explore Astrobiology!
Explore Astrobiology! Websites http://commtechlab.msu.edu/sites/dlc-me/zoo/ Visit the Microbe Zoo! Learn about microbes that live in soil, animals, food, space, and water and their important functions. Find out how microbes help us in our everyday lives and how they might be used on space missions and in space colonies. http://www.exploratorium.edu/origins/index.html The Exploratorium’s portal to websites that contain information on the people, explorations and theories involved in the search for life. http://www.lpi.usra.edu/education/timeline/ The Lunar and Planetary institute offers this site that provides the changes that occurred on Earth over the last 4 and a half billion years in timeline form. The timeline and all images are downloadable! Appropriate for all ages. http://nai.arc.nasa.gov/ NASA’s Astrobiology Institute Website offers feature stories, most recently answered questions and Alien Safari to help kids discover some of the most extreme organisms on our planet, and find out what they are telling astrobiologists about the search for life beyond Earth. Appropriate for ages 7 and up. http://quest.arc.nasa.gov/projects/astrobiology/astroventure/avhome.html NASA's Astro-Venture helps students ages 6 and up to explore NASA careers and astrobiology research by offering activities that allow them to "search for and build a planet with the necessary characteristics for human habitation." http://www.cellsalive.com/cam2.htm Watch bacteria grow in real time! This site offers images of bacteria growing over a period of time. Learn about the make-up of cells and play games offered by the site. -

Maximizing Specific Energy by Breeding Deuterium
Maximizing specific energy by breeding deuterium Justin Ball Ecole Polytechnique F´ed´eralede Lausanne (EPFL), Swiss Plasma Center (SPC), CH-1015 Lausanne, Switzerland E-mail: [email protected] Abstract. Specific energy (i.e. energy per unit mass) is one of the most fundamental and consequential properties of a fuel source. In this work, a systematic study of measured fusion cross-sections is performed to determine which reactions are potentially feasible and identify the fuel cycle that maximizes specific energy. This reveals that, by using normal hydrogen to breed deuterium via neutron capture, the conventional catalyzed D-D fusion fuel cycle can attain a specific energy greater than anything else. Simply surrounding a catalyzed D-D reactor with water enables deuterium fuel, the dominant stockpile of energy on Earth, to produce as much as 65% more energy. Lastly, the impact on space propulsion is considered, revealing that an effective exhaust velocity exceeding that of deuterium-helium-3 is theoretically possible. 1. Introduction The aim of this work is to investigate the basic question \What fuel source can provide the most energy with the least mass?" For this question, we will look into the near future and assume that the current incremental and steady development of technology will continue. However, we will not postulate any breakthroughs or new physical processes because they cannot be predicted with any certainty. It is already well-known that the answer to our question must involve fusion fuels [1,2], but nothing more precise has been rigorously establishedz. A more precise answer may be useful in the future arXiv:1908.00834v1 [physics.pop-ph] 2 Aug 2019 when the technology required to implement it becomes available.