Wnt Ligands Are Not Required for Planar Cell Polarity in the Drosophila Wing Or Notum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wnt Signaling in Vertebrate Neural Development and Function
J Neuroimmune Pharmacol (2012) 7:774–787 DOI 10.1007/s11481-012-9404-x INVITED REVIEW Wnt Signaling in Vertebrate Neural Development and Function Kimberly A. Mulligan & Benjamin N. R. Cheyette Received: 18 June 2012 /Accepted: 10 September 2012 /Published online: 27 September 2012 # Springer Science+Business Media, LLC 2012 Abstract Members of the Wnt family of secreted signaling immature neurons migrate to populate different cortical proteins influence many aspects of neural development and layers, nuclei, and ganglia in the forebrain, midbrain, hind- function. Wnts are required from neural induction and axis brain, and spinal cord. Each mature neuron elaborates an formation to axon guidance and synapse development, and axon and numerous dendrites while forming hundreds to even help modulate synapse activity. Wnt proteins activate a thousands of synapses with other neurons, enabling electro- variety of downstream signaling pathways and can induce a chemical communication throughout the nervous system. similar variety of cellular responses, including gene tran- All these developmental processes must be coordinated to scription changes and cytoskeletal rearrangements. This re- ensure proper construction and function of the CNS, and view provides an introduction to Wnt signaling pathways this requires the coordinated developmental activity of a and discusses current research on their roles in vertebrate vast array of genes. neural development and function. Members of the Wnt family of secreted signaling proteins are implicated in every step of neural development men- Keywords Wnt signaling . Neural development . tioned above. Wnt proteins provide positional information CNS patterning . Axon guidance . Dendrite growth . within the embryo for anterior-posterior axis specification of Synapse formation . -

Theranostics WNT6 Is a Novel Oncogenic Prognostic Biomarker In
Theranostics 2018, Vol. 8, Issue 17 4805 Ivyspring International Publisher Theranostics 2018; 8(17): 4805-4823. doi: 10.7150/thno.25025 Research Paper WNT6 is a novel oncogenic prognostic biomarker in human glioblastoma Céline S. Gonçalves1,2, Joana Vieira de Castro1,2, Marta Pojo1,2, Eduarda P. Martins1,2, Sandro Queirós1,2, Emmanuel Chautard3,4, Ricardo Taipa5, Manuel Melo Pires5, Afonso A. Pinto6, Fernando Pardal7, Carlos Custódia8, Cláudia C. Faria8,9, Carlos Clara10, Rui M. Reis1,2,10, Nuno Sousa1,2, Bruno M. Costa1,2 1. Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Campus Gualtar, 4710-057 Braga, Portugal 2. ICVS/3B’s - PT Government Associate Laboratory, Braga/Guimarães, Portugal 3. Université Clermont Auvergne, INSERM, U1240 IMoST, 63000 Clermont Ferrand, France 4. Pathology Department, Université Clermont Auvergne, Centre Jean Perrin, 63011 Clermont-Ferrand, France 5. Neuropathology Unit, Department of Neurosciences, Centro Hospitalar do Porto, Porto, Portugal 6. Department of Neurosurgery, Hospital Escala Braga, Sete Fontes - São Victor 4710-243 Braga, Portugal 7. Department of Pathology, Hospital Escala Braga, Sete Fontes - São Victor 4710-243 Braga, Portugal 8. Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal 9. Neurosurgery Department, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte (CHLN), Lisbon, Portugal 10. Molecular Oncology Research Center, Barretos Cancer Hospital, Barretos - S. Paulo, Brazil. Corresponding author: Bruno M. Costa, Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. Email: [email protected]; Phone: (+351)253604837; Fax: (+351)253604831 © Ivyspring International Publisher. -

Wnt-Independent and Wnt-Dependent Effects of APC Loss on the Chemotherapeutic Response
International Journal of Molecular Sciences Review Wnt-Independent and Wnt-Dependent Effects of APC Loss on the Chemotherapeutic Response Casey D. Stefanski 1,2 and Jenifer R. Prosperi 1,2,3,* 1 Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46617, USA; [email protected] 2 Mike and Josie Harper Cancer Research Institute, South Bend, IN 46617, USA 3 Department of Biochemistry and Molecular Biology, Indiana University School of Medicine-South Bend, South Bend, IN 46617, USA * Correspondence: [email protected]; Tel.: +1-574-631-4002 Received: 30 September 2020; Accepted: 20 October 2020; Published: 22 October 2020 Abstract: Resistance to chemotherapy occurs through mechanisms within the epithelial tumor cells or through interactions with components of the tumor microenvironment (TME). Chemoresistance and the development of recurrent tumors are two of the leading factors of cancer-related deaths. The Adenomatous Polyposis Coli (APC) tumor suppressor is lost in many different cancers, including colorectal, breast, and prostate cancer, and its loss correlates with a decreased overall survival in cancer patients. While APC is commonly known for its role as a negative regulator of the WNT pathway, APC has numerous binding partners and functional roles. Through APC’s interactions with DNA repair proteins, DNA replication proteins, tubulin, and other components, recent evidence has shown that APC regulates the chemotherapy response in cancer cells. In this review article, we provide an overview of some of the cellular processes in which APC participates and how they impact chemoresistance through both epithelial- and TME-derived mechanisms. Keywords: adenomatous polyposis coli; chemoresistance; WNT signaling 1. -

Evolutionarily Conserved Tbx5–Wnt2/2B Pathway Orchestrates Cardiopulmonary Development
Evolutionarily conserved Tbx5–Wnt2/2b pathway orchestrates cardiopulmonary development Jeffrey D. Steimlea,b,c, Scott A. Rankind,e,f,g, Christopher E. Slagleh,i,j,k, Jenna Bekenya,b,c, Ariel B. Rydeena,b,c, Sunny Sun-Kin Chanl,m, Junghun Kweona,b,c, Xinan H. Yanga,b,c, Kohta Ikegamia,b,c, Rangarajan D. Nadadura,b,c, Megan Rowtona,b,c, Andrew D. Hoffmanna,b,c, Sonja Lazarevica,b,c, William Thomasn,o, Erin A. T. Boyle Andersonp, Marko E. Horbn,o, Luis Luna-Zuritaq,r, Robert K. Hom, Michael Kybal,m, Bjarke Jensens, Aaron M. Zornd,e,f,g, Frank L. Conlonh,i,j,k, and Ivan P. Moskowitza,b,c,1 aDepartment of Pediatrics, University of Chicago, Chicago, IL 60637; bDepartment of Pathology, University of Chicago, Chicago, IL 60637; cDepartment of Human Genetics, University of Chicago, Chicago, IL 60637; dCenter for Stem Cell and Organoid Medicine, Cincinnati Children’s Research Foundation, Cincinnati, OH 45229; eDepartment of Pediatrics, College of Medicine, University of Cincinnati, Cincinnati, OH 45229; fDivision of Developmental Biology, Perinatal Institute, Cincinnati Children’s Research Foundation, Cincinnati Children’s Hospital Medical Center, University of Cincinnati, Cincinnati, OH 45229; gDepartment of Pediatrics, College of Medicine, University of Cincinnati, Cincinnati, OH 45229; hDepartment of Biology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; iDepartment of Genetics, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; jIntegrative Program for Biological and Genome Sciences, University of North -

REVIEW ARTICLE the Genetics of Autism
REVIEW ARTICLE The Genetics of Autism Rebecca Muhle, BA*; Stephanie V. Trentacoste, BA*; and Isabelle Rapin, MD‡ ABSTRACT. Autism is a complex, behaviorally de- tribution of a few well characterized X-linked disorders, fined, static disorder of the immature brain that is of male-to-male transmission in a number of families rules great concern to the practicing pediatrician because of an out X-linkage as the prevailing mode of inheritance. The astonishing 556% reported increase in pediatric preva- recurrence rate in siblings of affected children is ϳ2% to lence between 1991 and 1997, to a prevalence higher than 8%, much higher than the prevalence rate in the general that of spina bifida, cancer, or Down syndrome. This population but much lower than in single-gene diseases. jump is probably attributable to heightened awareness Twin studies reported 60% concordance for classic au- and changing diagnostic criteria rather than to new en- tism in monozygotic (MZ) twins versus 0 in dizygotic vironmental influences. Autism is not a disease but a (DZ) twins, the higher MZ concordance attesting to ge- syndrome with multiple nongenetic and genetic causes. netic inheritance as the predominant causative agent. By autism (the autistic spectrum disorders [ASDs]), we Reevaluation for a broader autistic phenotype that in- mean the wide spectrum of developmental disorders cluded communication and social disorders increased characterized by impairments in 3 behavioral domains: 1) concordance remarkably from 60% to 92% in MZ twins social interaction; 2) language, communication, and and from 0% to 10% in DZ pairs. This suggests that imaginative play; and 3) range of interests and activities. -

Deregulated Wnt/Β-Catenin Program in High-Risk Neuroblastomas Without
Oncogene (2008) 27, 1478–1488 & 2008 Nature Publishing Group All rights reserved 0950-9232/08 $30.00 www.nature.com/onc ONCOGENOMICS Deregulated Wnt/b-catenin program in high-risk neuroblastomas without MYCN amplification X Liu1, P Mazanek1, V Dam1, Q Wang1, H Zhao2, R Guo2, J Jagannathan1, A Cnaan2, JM Maris1,3 and MD Hogarty1,3 1Division of Oncology, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA; 2Department of Biostatistics and Epidemiology, University of Pennsylvania School of Medicine, Philadelphia, PA, USA and 3Department of Pediatrics, University of Pennsylvania School of Medicine, Philadelphia, PA, USA Neuroblastoma (NB) is a frequently lethal tumor of Introduction childhood. MYCN amplification accounts for the aggres- sive phenotype in a subset while the majority have no Neuroblastoma (NB) is a childhood embryonal malig- consistently identified molecular aberration but frequently nancy arising in the peripheral sympathetic nervous express MYC at high levels. We hypothesized that acti- system. Half of all children with NB present with features vated Wnt/b-catenin (CTNNB1) signaling might account that define their tumorsashigh riskwith poor overall for this as MYC is a b-catenin transcriptional target and survival despite intensive therapy (Matthay et al., 1999). multiple embryonal and neural crest malignancies have A subset of these tumors are characterized by high-level oncogenic alterations in this pathway. NB cell lines without genomic amplification of the MYCN proto-oncogene MYCN amplification express higher levels of MYC and (Matthay et al., 1999) but the remainder have no b-catenin (with aberrant nuclear localization) than MYCN- consistently identified aberration to account for their amplified cell lines. -

Towards an Integrated View of Wnt Signaling in Development Renée Van Amerongen and Roel Nusse*
HYPOTHESIS 3205 Development 136, 3205-3214 (2009) doi:10.1242/dev.033910 Towards an integrated view of Wnt signaling in development Renée van Amerongen and Roel Nusse* Wnt signaling is crucial for embryonic development in all animal Notably, components at virtually every level of the Wnt signal species studied to date. The interaction between Wnt proteins transduction cascade have been shown to affect both β-catenin- and cell surface receptors can result in a variety of intracellular dependent and -independent responses, depending on the cellular responses. A key remaining question is how these specific context. As we discuss below, this holds true for the Wnt proteins responses take shape in the context of a complex, multicellular themselves, as well as for their receptors and some intracellular organism. Recent studies suggest that we have to revise some of messengers. Rather than concluding that these proteins are shared our most basic ideas about Wnt signal transduction. Rather than between pathways, we instead propose that it is the total net thinking about Wnt signaling in terms of distinct, linear, cellular balance of signals that ultimately determines the response of the signaling pathways, we propose a novel view that considers the receiving cell. In the context of an intact and developing integration of multiple, often simultaneous, inputs at the level organism, cells receive multiple, dynamic, often simultaneous and of both Wnt-receptor binding and the downstream, sometimes even conflicting inputs, all of which are integrated to intracellular response. elicit the appropriate cell behavior in response. As such, the different signaling pathways might thus be more intimately Introduction intertwined than previously envisioned. -

Multi-Functionality of Proteins Involved in GPCR and G Protein Signaling: Making Sense of Structure–Function Continuum with In
Cellular and Molecular Life Sciences (2019) 76:4461–4492 https://doi.org/10.1007/s00018-019-03276-1 Cellular andMolecular Life Sciences REVIEW Multi‑functionality of proteins involved in GPCR and G protein signaling: making sense of structure–function continuum with intrinsic disorder‑based proteoforms Alexander V. Fonin1 · April L. Darling2 · Irina M. Kuznetsova1 · Konstantin K. Turoverov1,3 · Vladimir N. Uversky2,4 Received: 5 August 2019 / Revised: 5 August 2019 / Accepted: 12 August 2019 / Published online: 19 August 2019 © Springer Nature Switzerland AG 2019 Abstract GPCR–G protein signaling system recognizes a multitude of extracellular ligands and triggers a variety of intracellular signal- ing cascades in response. In humans, this system includes more than 800 various GPCRs and a large set of heterotrimeric G proteins. Complexity of this system goes far beyond a multitude of pair-wise ligand–GPCR and GPCR–G protein interactions. In fact, one GPCR can recognize more than one extracellular signal and interact with more than one G protein. Furthermore, one ligand can activate more than one GPCR, and multiple GPCRs can couple to the same G protein. This defnes an intricate multifunctionality of this important signaling system. Here, we show that the multifunctionality of GPCR–G protein system represents an illustrative example of the protein structure–function continuum, where structures of the involved proteins represent a complex mosaic of diferently folded regions (foldons, non-foldons, unfoldons, semi-foldons, and inducible foldons). The functionality of resulting highly dynamic conformational ensembles is fne-tuned by various post-translational modifcations and alternative splicing, and such ensembles can undergo dramatic changes at interaction with their specifc partners. -

WNT2 and WNT7B Cooperative Signaling in Lung Development
University of Pennsylvania ScholarlyCommons Publicly Accessible Penn Dissertations 2012 WNT2 and WNT7B Cooperative Signaling in Lung Development Mayumi Miller University of Pennsylvania, [email protected] Follow this and additional works at: https://repository.upenn.edu/edissertations Part of the Developmental Biology Commons, and the Molecular Biology Commons Recommended Citation Miller, Mayumi, "WNT2 and WNT7B Cooperative Signaling in Lung Development" (2012). Publicly Accessible Penn Dissertations. 674. https://repository.upenn.edu/edissertations/674 This paper is posted at ScholarlyCommons. https://repository.upenn.edu/edissertations/674 For more information, please contact [email protected]. WNT2 and WNT7B Cooperative Signaling in Lung Development Abstract The development of a complex organ, such as the lung, relies upon precisely controlled temporal and spatial expression patterns of signaling pathways for proper specification and differentiation of the cell types required to build a lung. While progress has been made in dissecting the network of signaling pathways and the integration of their positive and negative feedback mechanisms, there is still much to discover. For example, the Wnt signaling pathway is required for lung specification and growth, but a combinatorial role for Wnt ligands has not been investigated. In this dissertation, I combine mouse genetic models and in vitro and ex vivo lung culture assays, to determine a cooperative role for Wnt2 and Wnt7b in the developing lung. This body of work reveals the requirement of cooperative signaling between Wnt2 and Wnt7b for smooth muscle development and proximal to distal patterning of the lung. Additional findings er veal a role for the Pdgf pathway and homeobox genes in potentiating this cooperation. -

Wnt4/B2catenin Signaling in Medullary Kidney Myofibroblasts
BASIC RESEARCH www.jasn.org Wnt4/b2Catenin Signaling in Medullary Kidney Myofibroblasts † †‡ | Derek P. DiRocco,* Akio Kobayashi,* Makoto M. Taketo,§ Andrew P. McMahon, and †‡ Benjamin D. Humphreys* *Renal Division, Brigham and Women’s Hospital, Boston, Massachusetts; †Harvard Medical School, Boston, Massachusetts; ‡Harvard Stem Cell Institute, Cambridge, Massachusetts; §Department of Pharmacology, Graduate School of Medicine, Kyoto University, Yoshida-Konoé-cho, Sakyo, Kyoto, Japan; and |Department of Stem Cell Biology and Regenerative Medicine, Eli and Edythe Broad-CIRM Center for Regenerative Medicine and Stem Cell Research, Keck School of Medicine of the University of Southern California, Los Angeles, California ABSTRACT Injury to the adult kidney induces a number of developmental genes thought to regulate repair, including Wnt4. During kidney development, early nephron precursors and medullary stroma both express Wnt4, where it regulates epithelialization and controls smooth muscle fate, respectively. Expression patterns and roles for Wnt4 in the adult kidney, however, remain unclear. In this study, we used reporters, lineage analysis, and conditional knockout or activation of the Wnt/b-catenin pathway to investigate Wnt4 in the adult kidney. Proliferating, medullary, interstitial myofibroblasts strongly expressed Wnt4 during renal fibrosis, whereas tubule epithelia, except for the collecting duct, did not. Exogenous Wnt4 drove myofi- broblast differentiation of a pericyte-like cell line, suggesting that Wnt4 might regulate pericyte-to-myo- fibroblast transition through autocrine signaling. However, conditional deletion of Wnt4 in interstitial cells did not reduce myofibroblast proliferation, cell number, or myofibroblast gene expression during fibrosis. Because the injured kidney expresses multiple Wnt ligands that might compensate for the absence of Wnt4, we generated a mouse model with constitutive activation of canonical Wnt/b-catenin signaling in interstitial pericytes and fibroblasts. -

The WNT10A Gene in Ectodermal Dysplasias and Selective Tooth Agenesis Gabriele Mues,1* John Bonds,1 Lilin Xiang,1 Alexandre R
RESEARCH ARTICLE The WNT10A Gene in Ectodermal Dysplasias and Selective Tooth Agenesis Gabriele Mues,1* John Bonds,1 Lilin Xiang,1 Alexandre R. Vieira,2 Figen Seymen,3 Ophir Klein,4,5,6 and Rena N. D’Souza1 1Department of Biomedical Sciences, Texas A&M University-HSC Baylor College of Dentistry, Dallas, Texas 2Department of Oral Biology, University of Pittsburgh School of Dental Medicine, Pittsburgh, Pennsylvania 3University of Istanbul Faculty of Dentistry, Istanbul, Turkey 4Department of Orofacial Sciences, University of California San Francisco, San Francisco, California 5Department of Pediatrics, University of California San Francisco, San Francisco, California 6Institute for Human Genetics and Regeneration Medicine, University of California San Francisco, San Francisco, California Manuscript Received: 8 October 2013; Manuscript Accepted: 30 January 2014 Mutations in the WNT10A gene were first detected in the rare syndrome odonto-onycho-dermal dysplasia (OODD, How to Cite this Article: OMIM257980) but have now also been found to cause about Mues G, Bonds J, Xiang L, Vieira AR, 35–50% of selective tooth agenesis (STHAG4, OMIM150400), a Seymen F, Klein O, D’Souza RN. 2014. common disorder that mostly affects the permanent dentition. The WNT10A gene in ectodermal In our random sample of tooth agenesis patients, 40% had at least dysplasias and selective tooth agenesis. one mutation in the WNT10A gene. The WNT10A Phe228Ile variant alone reached an allele frequency of 0.21 in the tooth Am J Med Genet Part A 164A:2455–2460. agenesis cohort, about 10 times higher than the allele frequency reported in large SNP databases for Caucasian populations. Patients with bi-allelic WNT10A mutations have severe tooth syndrome (OMIM 224750) which additionally features eyelid cysts agenesis while heterozygous individuals are either unaffected or and predisposition to adnexal skin tumors. -
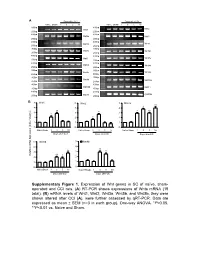
Supplementary Figure 1. Expression of Wnt Genes in SC of Naïve
A Days after CCI Days after CCI Naive Sham 1 3 5 10 Naive Sham 1 3 5 10 500bp 400bp Wnt1 Wnt2 250bp 250bp 400bp 400bp Wnt2b Wnt3 250bp 250bp 400bp 400bp Wnt3a Wnt4 250bp 250bp 300bp 400bp Wnt5a Wnt5b 200bp 250bp 400bp 400bp Wnt6 Wnt7a 250bp 250bp 400bp 400bp Wnt7b Wnt8a 250bp 250bp 400bp 350bp Wnt8b Wnt9a 250bp 200bp 400bp 400bp Wnt9b Wnt10a 250bp 250bp 400bp 400bp Wnt10b Wnt11 250bp 250bp 400bp 400bp Wnt16 GAPDH 250bp 250bp B 5 Wnt1 5 Wnt2 5 Wnt3a ** 4 4 4 * ** ** 3 3 * 3 * * 2 2 * 2 1 1 1 0 0 0 NaïveSham 1 3 5 10 Naïve Sham 1 3 5 10 Naïve Sham 1 3 5 10 Days after CCI Days after CCI Days after CCI 5 Wnt5b 5 Wnt8b 4 4 ** 3 ** 3 ** Relative mRNA Expression (fold of change) ** 2 * 2 1 1 0 0 NaïveSham 1 3 5 10 NaïveSham 1 3 5 10 Days after CCI Days after CCI Supplementary Figure 1 A B Negative Weak Moderate Strong Negative Weak Moderate Strong Large-sized cells 100% 100% 80% 80% 60% 60% 40% 20% 40% 0% 20% Sham CCI-1d CCI-14d Medium-sized cells Wnt3a immunoreactivity cells 0% 100% CGRP(+) IB4(+) 80% Small cells 60% 40% 20% 0% Sham CCI-1d CCI-14d Wnt3a immunoreactivity cells Small cells 100% 80% 60% 40% 20% 0% Sham CCI-1d CCI-14d Fig. S2 4 Fz1 4 Fz3 4 Fz4 4 Fz5 ** 3 3 ** 3 3 2 ** 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1 5 10 NaïveSham 1 5 10 Days after CCI Days after CCI Days after CCI Days after CCI 4 Fz6 4 Fz7 4 Fz8 4 Fz9 (Fold of Change) of (Fold 3 3 3 ** 3 Relative mRNA Expression Expression mRNA Relative ** 2 2 2 ** 2 * 1 1 1 1 0 0 0 0 NaïveSham 1 5 10 NaïveSham 1 5 10 Naïve Sham 1d 5d 10d NaïveSham 1