Vaccine Hesitancy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Hepatitis B Vaccine Is the Most Widely Used Vaccine in the World, with Over 1 Billion Doses Given
Hepatitis B Vaccine Protect Yourself and Those You Love What is Hepatitis B? Hepatitis B is the most common serious liver infection in the world. It is caused by the hepatitis B virus (HBV), which attacks liver cells and can lead to liver failure, cirrhosis (scarring), or liver cancer later in life. The virus is transmitted through direct contact with infected blood and bodily fluids, and from an infected woman to her newborn at birth. Is there a safe vaccine for hepatitis B? YES! The good news is that there is a safe and effective vaccine for hepatitis B. The vaccine is a series, typically given as three shots over a six-month period that will provide a lifetime of protection. You cannot get hepatitis B from the vaccine – there is no human blood or live virus in the vaccine. The hepatitis B vaccine is the most widely used vaccine in the world, with over 1 billion doses given. The hepatitis B vaccine is the first "anti-cancer" vaccine because it can help prevent liver cancer! Who should be vaccinated against hepatitis B? The World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) recommend the hepatitis B vaccine for all newborns and children up to 18 years of age, and all high-risk adults. All infants should receive the first dose of the vaccine in the delivery room or in the first 24 hours of life, preferably within 12 hours. (CDC recommends the first dose within 12 hours vs. the WHO recommendation of 24 hours.) The HBV vaccine is recommended to anyone who is at high risk of infection. -
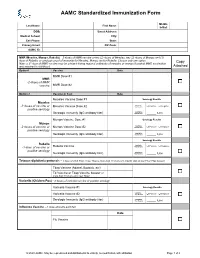
AAMC Standardized Immunization Form
AAMC Standardized Immunization Form Middle Last Name: First Name: Initial: DOB: Street Address: Medical School: City: Cell Phone: State: Primary Email: ZIP Code: AAMC ID: MMR (Measles, Mumps, Rubella) – 2 doses of MMR vaccine or two (2) doses of Measles, two (2) doses of Mumps and (1) dose of Rubella; or serologic proof of immunity for Measles, Mumps and/or Rubella. Choose only one option. Copy Note: a 3rd dose of MMR vaccine may be advised during regional outbreaks of measles or mumps if original MMR vaccination was received in childhood. Attached Option1 Vaccine Date MMR Dose #1 MMR -2 doses of MMR vaccine MMR Dose #2 Option 2 Vaccine or Test Date Measles Vaccine Dose #1 Serology Results Measles Qualitative -2 doses of vaccine or Measles Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Mumps Vaccine Dose #1 Serology Results Mumps Qualitative -2 doses of vaccine or Mumps Vaccine Dose #2 Titer Results: Positive Negative positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Serology Results Rubella Qualitative Positive Negative -1 dose of vaccine or Rubella Vaccine Titer Results: positive serology Quantitative Serologic Immunity (IgG antibody titer) Titer Results: _____ IU/ml Tetanus-diphtheria-pertussis – 1 dose of adult Tdap; if last Tdap is more than 10 years old, provide date of last Td or Tdap booster Tdap Vaccine (Adacel, Boostrix, etc) Td Vaccine or Tdap Vaccine booster (if more than 10 years since last Tdap) Varicella (Chicken Pox) - 2 doses of varicella vaccine or positive serology Varicella Vaccine #1 Serology Results Qualitative Varicella Vaccine #2 Titer Results: Positive Negative Serologic Immunity (IgG antibody titer) Quantitative Titer Results: _____ IU/ml Influenza Vaccine --1 dose annually each fall Date Flu Vaccine © 2020 AAMC. -

Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC)
AAMC Standardized Immunization Form 2020 Hepatitis B Vaccine – Frequently Asked Questions (Information from the CDC) 1. What are the hepatitis B vaccines licensed for use in the United States? Three single-antigen vaccines and two combination vaccines are currently licensed in the United States. Single-antigen hepatitis B vaccines: • ENGERIX-B® • RECOMBIVAX HB® • HEPLISAV-B™ Combination vaccines: • PEDIARIX®: Combined hepatitis B, diphtheria, tetanus, acellular pertussis (DTaP), and inactivated poliovirus (IPV) vaccine. Cannot be administered before age 6 weeks or after age 7 years. • TWINRIX®: Combined Hepatitis A and hepatitis B vaccine. Recommended for people aged ≥18 years who are at increased risk for both HAV and HBV infections. 2. What are the recommended schedules for hepatitis B vaccination? The vaccination schedule most often used for children and adults is three doses given at 0, 1, and 6 months. Alternate schedules have been approved for certain vaccines and/or populations. A new formulation, Heplisav-B (HepB-CpG), is approved to be given as two doses one month apart. 3. If there is an interruption between doses of hepatitis B vaccine, does the vaccine series need to be restarted? No. The series does not need to be restarted but the following should be considered: • If the vaccine series was interrupted after the first dose, the second dose should be administered as soon as possible. • The second and third doses should be separated by an interval of at least 8 weeks. • If only the third dose is delayed, it should be administered as soon as possible. 4. Is it harmful to administer an extra dose of hepatitis B vaccine or to repeat the entire vaccine series if documentation of the vaccination history is unavailable or the serology test is negative? No, administering extra doses of single-antigen hepatitis B vaccine is not harmful. -

COVID-19 Vaccines Frequently Asked Questions
Page 1 of 12 COVID-19 Vaccines 2020a Frequently Asked Questions Michigan.gov/Coronavirus The information in this document will change frequently as we learn more about COVID-19 vaccines. There is a lot we are learning as the pandemic and COVID-19 vaccines evolve. The approach in Michigan will adapt as we learn more. September 29, 2021. Quick Links What’s new | Why COVID-19 vaccination is important | Booster and additional doses | What to expect when you get vaccinated | Safety of the vaccine | Vaccine distribution/prioritization | Additional vaccine information | Protecting your privacy | Where can I get more information? What’s new − Pfizer booster doses recommended for some people to boost waning immunity six months after completing the Pfizer vaccine. Why COVID-19 vaccination is important − If you are fully vaccinated, you don’t have to quarantine after being exposed to COVID-19, as long as you don’t have symptoms. This means missing less work, school, sports and other activities. − COVID-19 vaccination is the safest way to build protection. COVID-19 is still a threat, especially to people who are unvaccinated. Some people who get COVID-19 can become severely ill, which could result in hospitalization, and some people have ongoing health problems several weeks or even longer after getting infected. Even people who did not have symptoms when they were infected can have these ongoing health problems. − After you are fully vaccinated for COVID-19, you can resume many activities that you did before the pandemic. CDC recommends that fully vaccinated people wear a mask in public indoor settings if they are in an area of substantial or high transmission. -

Immunogenicity and Safety of a Third Dose, and Immune Persistence Of
medRxiv preprint doi: https://doi.org/10.1101/2021.07.23.21261026; this version posted July 25, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. 1 Immunogenicity and safety of a third dose, and immune persistence of 2 CoronaVac vaccine in healthy adults aged 18-59 years: interim results 3 from a double-blind, randomized, placebo-controlled phase 2 clinical 4 trial 5 6 Hongxing Pan MSc1*, Qianhui Wu MPH2*, Gang Zeng Ph.D.3*, Juan Yang Ph.D.1, Deyu 7 Jiang MSc4, Xiaowei Deng MSc2, Kai Chu MSc1, Wen Zheng BSc2, Fengcai Zhu M.D.5†, 8 Hongjie Yu M.D. Ph.D.2,6,7†, Weidong Yin MBA8† 9 10 Affiliations 11 1. Vaccine Evaluation Institute, Jiangsu Provincial Center for Disease Control and 12 Prevention, Nanjing, China 13 2. School of Public Health, Fudan University, Key Laboratory of Public Health Safety, 14 Ministry of Education, Shanghai, China 15 3. Clinical Research Department, Sinovac Biotech Co., Ltd., Beijing, China 16 4. Covid-19 Vaccine Department, Sinovac Life Sciences Co., Ltd., Beijing, China 17 5. Jiangsu Provincial Center for Disease Control and Prevention, Nanjing, China 18 6. Shanghai Institute of Infectious Disease and Biosecurity, Fudan University, 19 Shanghai, China 20 7. Department of Infectious Diseases, Huashan Hospital, Fudan University, 21 Shanghai, China 22 8. Sinovac Biotech Co., Ltd., Beijing, China NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. -

The Mississippi Covid-19 Vaccine Confidence Survey: Population Results Report
MAY 2021 THE MISSISSIPPI COVID-19 VACCINE CONFIDENCE SURVEY: POPULATION RESULTS Report A collaborative population-based study The Mississippi Community Engagement Alliance Against COVID-19 Disparities (CEAL) Team The Mississippi State Department of Health: Office of Preventive Health and Health Equity EXECUTIVE SUMMARY Since the Spring of 2020, the Novel 2019 Office of Preventive Health and Health Coronavirus (COVID-19) Pandemic has Equity (OPHHE) disseminated a statewide impacted Mississippians of every race, vaccine confidence survey beginning end ethnicity, age, gender, and income bracket. of December 2020 and collecting data Unfortunately, it has disproportionately until March 2021. The survey is intended to impacted Mississippians of color, the elderly, be representative of Mississippians, with and those living with chronic disease. For intentional efforts invested to reach lower most of the past year, the State has worked income and rural Mississippi populations, as to protect its population through preventive well as the state’s Black, Hispanic (Latino/ measures such as social distancing and Latinx), Asian (including the Vietnamese personal protective equipment. However, population of the Gulf Coast), and Native with the release of COVID-19 Vaccines to American/Choctaw communities. The sur- the public, the population of Mississippi has vey was administered in three languages- the opportunity to embrace a long-term English, Spanish, and Vietnamese- through a solution to COVID-19. That is, if Mississippians mixed-modal survey effort, including: web- are willing to receive the vaccine. To based, paper-based, and verbal-oratory assess Mississippians’ COVID-19 Vaccine administration. All targeted populations confidence, the Mississippi Community were ultimately reached and are represented Engagement Alliance Against COVID-19 in the over 11,000 completed responses Disparities (CEAL) Team and the Mississippi from all 82 of Mississippi’s counties. -

Immunity and How Vaccines Work
Immunity and how vaccines work Dr Brenda Corcoran National Immunisation Office Presentation Outline An understanding of the following principles: • Overview of immunity • Different types of vaccines and vaccine contents • Vaccine failures • Time intervals between vaccine doses • Vaccine overload • Adverse reactions • Herd immunity Immunity Immunity • The ability of the human body to protect itself from infectious disease The immune system • Cells with a protective function in the – bone marrow – thymus – lymphatic system of ducts and nodes – spleen –blood Types of immunity Source: http://en.wikipedia.org/wiki/Immunological_memory Natural (innate) immunity Non-specific mechanisms – Physical barriers • skin and mucous membranes – Chemical barriers • gastric and digestive enzymes – Cellular and protein secretions • phagocytes, macrophages, complement system ** No “memory” of protection exists afterwards ** Passive immunity – adaptive mechanisms Natural • maternal transfer of antibodies to infant via placenta Artificial • administration of pre- formed substance to provide immediate but short-term protection (antitoxin, antibodies) Protection is temporary and wanes with time (usually few months) Active immunity – adaptive mechanisms Natural • following contact with organism Artificial • administration of agent to stimulate immune response (immunisation) Acquired through contact with an micro-organism Protection produced by individual’s own immune system Protection often life-long but may need boosting How vaccines work • Induce active immunity – Immunity and immunologic memory similar to natural infection but without risk of disease • Immunological memory allows – Rapid recognition and response to pathogen – Prevent or modify effect of disease Live attenuated vaccines Weakened viruses /bacteria – Achieved by growing numerous generations in laboratory – Produces long lasting immune response after one or two doses – Stimulates immune system to react as it does to natural infection – Can cause mild form of the disease (e.g. -

Pfizer-Biontech COVID-19 Vaccine Have the Same Formulation and Can Be Used Interchangeably to Provide the COVID-19 Vaccination Series
PRODUCT MONOGRAPH INCLUDING PATIENT MEDICATION INFORMATION COMIRNATY COVID-19 Vaccine, mRNA Suspension for Intramuscular Injection Multiple Dose Vial (after dilution each vial contains 6† doses of 0.3 mL) Active Immunizing Agent BioNTech Manufacturing GmbH Date of Initial Authorization: An der Goldgrube 12 September 16, 2021 Mainz, Rhineland-Palatinate, Germany 55131 Imported and distributed by: Pfizer Canada ULC 17,300 Trans-Canada Highway Kirkland, Quebec, Canada H9J 2M5 Submission Control Number: 252736 † Low dead-volume syringes and/or needles can be used to extract 6 doses from a single vial. If standard syringes and needles are used, there may not be sufficient volume to extract a 6th dose from a single vial. COMIRNATY (COVID-19 Vaccine, mRNA) Product Monograph Page 1 of 33 TABLE OF CONTENTS Sections or subsections that are not applicable at the time of authorization are not listed. TABLE OF CONTENTS ............................................................................................................ 2 PART I: HEALTH PROFESSIONAL INFORMATION .................................................................... 4 1 INDICATIONS ............................................................................................................. 4 1.1 Pediatrics .................................................................................................................. 4 1.2 Geriatrics .................................................................................................................. 4 2 CONTRAINDICATIONS ............................................................................................... -

Standing Orders for Administering Hepatitis B Vaccine to Adults
Standing orders for other vaccines are available at www.immunize.org/standing-orders. note: This standing orders template may be adapted per a practice’s discretion without obtaining permission from IAC. As a courtesy, please acknowledge IAC as its source. standing orders for Administering Hepatitis B Vaccine to Adults Purpose To reduce morbidity and mortality from hepatitis B virus (HBV) by vaccinating all adults who meet the criteria estab- lished by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices. Policy Where allowed by state law, standing orders enable eligible nurses and other health care professionals (e.g., pharma- cists) to assess the need for vaccination and to vaccinate adults who meet any of the criteria below. Procedure 1 Assess Adults for Need of Vaccination against HBV infection according to the following criteria: • Any person who wants to be protected from HBV infection • Patient with diabetes mellitus (Note: for those age 60 years or older with diabetes mellitus, at the discretion of the treating clinician) • Patient with end-stage renal disease, including patients receiving hemodialysis; HIV infection; or chronic liver disease • Sexually active and not in a long-term, mutually monogamous relationship (e.g., more than 1 sex partner during the previous 6 months) • Seeking evaluation or receiving treatment for a sexually transmitted infection (STI) • A male who has sex with males • A current or recent injection-drug user • At occupational risk of infection through exposure to blood or blood-contaminated body fluids (e.g., healthcare worker, public safety worker, trainee in a health professional or allied health school) • Residents or staff of an institution for persons with developmental disabilities • Sex partner or household member of a person who is chronically infected with HBV (HBsAg-positive). -

COVID-19: Mechanisms of Vaccination and Immunity
Review COVID-19: Mechanisms of Vaccination and Immunity Daniel E. Speiser 1,* and Martin F. Bachmann 2,3,4,* 1 Department of Oncology, University Hospital and University of Lausanne, 1066 Lausanne, Switzerland 2 International Immunology Centre, Anhui Agricultural University, Hefei 230036, China 3 Department of Rheumatology, Immunology and Allergology, Inselspital, University of Bern, 3010 Bern, Switzerland 4 Department of BioMedical Research, University of Bern, 3008 Bern, Switzerland * Correspondence: [email protected] (D.E.S.); [email protected] (M.F.B.) Received: 2 July 2020; Accepted: 20 July 2020; Published: 22 July 2020 Abstract: Vaccines are needed to protect from SARS-CoV-2, the virus causing COVID-19. Vaccines that induce large quantities of high affinity virus-neutralizing antibodies may optimally prevent infection and avoid unfavorable effects. Vaccination trials require precise clinical management, complemented with detailed evaluation of safety and immune responses. Here, we review the pros and cons of available vaccine platforms and options to accelerate vaccine development towards the safe immunization of the world’s population against SARS-CoV-2. Favorable vaccines, used in well-designed vaccination strategies, may be critical for limiting harm and promoting trust and a long-term return to normal public life and economy. Keywords: SARS-CoV-2; COVID-19; nucleic acid tests; serology; vaccination; immunity 1. Introduction The COVID-19 pandemic holds great challenges for which the world is only partially prepared [1]. SARS-CoV-2 combines serious pathogenicity with high infectivity. The latter is enhanced by the fact that asymptomatic and pre-symptomatic individuals can transmit the virus, in contrast to SARS-CoV-1 and MERS-CoV, which were transmitted by symptomatic patients and could be contained more efficiently [2,3]. -

Recommended Composition of Influenza Virus Vaccines for Use in the 2021- 2022 Northern Hemisphere Influenza Season
Recommended composition of influenza virus vaccines for use in the 2021- 2022 northern hemisphere influenza season February 2021 WHO convenes technical consultations1 in February and September each year to recommend viruses for inclusion in influenza vaccines 2 for the northern and southern hemisphere influenza seasons, respectively. This recommendation relates to the influenza vaccines for use in the northern hemisphere 2021-2022 influenza season. A recommendation will be made in September 2021 relating to vaccines that will be used for the southern hemisphere 2022 influenza season. For countries in tropical and subtropical regions, WHO recommendations for influenza vaccine composition (northern hemisphere or southern hemisphere) are available on the WHO Global Influenza Programme website3. Seasonal influenza activity Public health and laboratory responses to the COVID-19 pandemic, caused by the coronavirus SARS- CoV-2, initially led to reduced influenza surveillance and/or reporting activities in many countries, which have been improving. Additionally, COVID-19 mitigation strategies including restrictions on travel, use of respiratory protection, and social-distancing measures in most countries have contributed to decreased influenza activity. Overall, record-low levels of influenza detections were reported and fewer viruses were available for characterization during the September 2020 to January 2021 time- period than in previous years. Between September 2020 and January 2021, influenza A(H1N1)pdm09, A(H3N2) and influenza B viruses circulated in very low numbers and the relative proportions of the viruses circulating varied among reporting countries. Globally, since September 2020, influenza activity was mostly reported from countries in the tropics and subtropics and some countries in the temperate zone of the northern hemisphere. -

Vaccine Report
COVID-19: vaccine summary Vaccination data through Jun 2, 2021 as of Jun 3, 2021 at 12:05 AM Data in this report are provisional and subject to change. Data in this summary pertain to COVID-19 vaccines approved by the U.S. Food and Drug Administration and have been issued an Emergency Use Authorization (Janssen COVID-19 Vaccine, Moderna COVID-19 Vaccine and Pfizer-BioNTech COVID-19 Vaccine). These data summarize the number of people who have received either their first dose or have completed the series for a COVID-19 vaccine. A person can only be counted in one category, first dose or series complete. For persons receiving Moderna or Pfizer vaccines, once they receive their second dose, they are moved from the first dose column to the series completed column. Because of this, the total first dose category may show a change from report to report not matching the total receiving their first dose from the previous day. Persons receiving Johnson & Johnson vaccines will always be counted in the series completed column. Series Total people COVID-19 doses for vaccinated persons in Florida Demographic summary First dose complete vaccinated Doses 17,967,048 Age group 2,004,239 8,393,060 10,397,299 People receiving one dose of a 1-dose series (Johnson & Johnson) or receiving only 12-14 years 91,161 410 91,571 one dose of a 2-dose series (Moderna, Pfizer) count once. Persons receiving both 15-24 years 255,486 519,348 774,834 doses of a 2-dose series count twice.