Lolldaiga Hills Research Programme
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chinko/Mbari Drainage Basin Represents a Conservation Hotspot for Eastern Derby Eland in Central Africa
Published in "African Journal of Ecology 56 (2): 194–201, 2018" which should be cited to refer to this work. Chinko/Mbari drainage basin represents a conservation hotspot for Eastern Derby eland in Central Africa Karolına Brandlova1 | Marketa Glonekova1 | Pavla Hejcmanova1 | Pavla Jůnkova Vymyslicka1 | Thierry Aebischer2 | Raffael Hickisch3 | David Mallon4 1Faculty of Tropical AgriSciences, Czech University of Life Sciences Prague, Praha 6, Abstract Czech Republic One of the largest of antelopes, Derby eland (Taurotragus derbianus), is an important 2Department of Biology, University of ecosystem component of African savannah. While the western subspecies is Critically Fribourg, Fribourg, Switzerland 3Chinko Project, Operations, Chinko, Endangered, the eastern subspecies is classified as least concern. Our study presents the Bangui, Central African Republic first investigation of population dynamics of the Derby eland in the Chinko/Mbari Drai- 4 Co-Chair, IUCN/SSC Antelope Specialist nage Basin, Central African Republic, and assesses the conservation role of this popula- Group and Division of Biology and Conservation Ecology, Manchester tion. We analysed data from 63 camera traps installed in 2012. The number of individuals Metropolitan University, Glossop, UK captured within a single camera event ranged from one to 41. Herds were mostly mixed Correspondence by age and sex, mean group size was 5.61, larger during the dry season. Adult (AD) males ı Karol na Brandlova constituted only 20% of solitary individuals. The overall sex ratio (M:F) was 1:1.33, while Email: [email protected] the AD sex ratio shifted to 1:1.52, reflecting selective hunting pressure. Mean density Funding information ranged from 0.04 to 0.16 individuals/km2, giving an estimated population size of 445– Czech University of Life Sciences Prague, Grant/Award Number: 20135010, 1,760 individuals. -

Animals of Africa
Silver 49 Bronze 26 Gold 59 Copper 17 Animals of Africa _______________________________________________Diamond 80 PYGMY ANTELOPES Klipspringer Common oribi Haggard oribi Gold 59 Bronze 26 Silver 49 Copper 17 Bronze 26 Silver 49 Gold 61 Copper 17 Diamond 80 Diamond 80 Steenbok 1 234 5 _______________________________________________ _______________________________________________ Cape grysbok BIG CATS LECHWE, KOB, PUKU Sharpe grysbok African lion 1 2 2 2 Common lechwe Livingstone suni African leopard***** Kafue Flats lechwe East African suni African cheetah***** _______________________________________________ Red lechwe Royal antelope SMALL CATS & AFRICAN CIVET Black lechwe Bates pygmy antelope Serval Nile lechwe 1 1 2 2 4 _______________________________________________ Caracal 2 White-eared kob DIK-DIKS African wild cat Uganda kob Salt dik-dik African golden cat CentralAfrican kob Harar dik-dik 1 2 2 African civet _______________________________________________ Western kob (Buffon) Guenther dik-dik HYENAS Puku Kirk dik-dik Spotted hyena 1 1 1 _______________________________________________ Damara dik-dik REEDBUCKS & RHEBOK Brown hyena Phillips dik-dik Common reedbuck _______________________________________________ _______________________________________________African striped hyena Eastern bohor reedbuck BUSH DUIKERS THICK-SKINNED GAME Abyssinian bohor reedbuck Southern bush duiker _______________________________________________African elephant 1 1 1 Sudan bohor reedbuck Angolan bush duiker (closed) 1 122 2 Black rhinoceros** *** Nigerian -

On the Use of the IUCN Status for the Management of Trophy Hunting
CSIRO PUBLISHING Wildlife Research, 2012, 39, 711–720 http://dx.doi.org/10.1071/WR12121 On the use of the IUCN status for the management of trophy hunting Lucille Palazy A,B,C, Christophe Bonenfant A, Jean-Michel Gaillard A and Franck Courchamp B ALaboratoire Biométrie et Biologie Évolutive, UMR CNRS 5558, University Claude Bernard Lyon 1, 43 boulevard du 11 novembre 1918, 69622 Villeurbanne Cedex, France. BLaboratoire Écologie, Systématique et Evolution, UMR CNRS 8079, University Paris Sud, 91405 Orsay Cedex, France. CCorresponding author. Email: [email protected] Abstract Context. Whether trophy hunting is beneficial or a threat to the conservation of species is an open and hotly debated question. The International Union for Conservation of Nature (IUCN) is in charge of assessing the need for species protection at the global scale and providing a useful guide for sustainable exploitation and conservation. Consideration of the IUCN status in wildlife management and its consequences on the attractiveness of trophy-hunted species remains to be quantified. Aims. The present study investigated the link between the IUCN status of the trophy species and its exploitation in 124 taxa. We expected that the number of trophies should be inversely correlated with the IUCN vulnerability status across species. Methods. Using the database of the Safari Club International, one of the largest hunting associations worldwide, we investigated the effect (1) of the first status attribution and (2) of an upgrade of the IUCN status on the number of trophies recorded by the Safari Club International, by comparing the average number of trophies 5 years before and after a status change. -

The Distribution of Some Large Mammals in Kenya Staff, White Hunters, Foresters, Agricultural and Veterinary Officers, Farmers and Many Others
ACKNOWLEDGEMENTS The publication of this paper would not have been possible without the generous support of the following: The International Union for Conservation of Nature and Natural Resources. The World Wildlife Fund. Monsieur Charles Vander Elst. J.E.Afr.Nat.Hist.Soc. Vol.XXIV No.3 (107) June 1963 Introduction Detailed distribution maps of wild animals play a useful part in the study of the ecology and status of the species concerned, and form a basis for comparison in future years. They are also a valu• able aid to the sound planning of conservation and exploitation measures affecting wild life. The distribution maps of large mammals presented in this paper have been prepared as part of the programme of the Fauna Research Unit of the Kenya Game Department. They refer only to the distribution of the species within Kenya, although it would be desirable when possible to extend the work beyond this biologically meaningless boundary. We have dealt only with the pachyderms and the larger carnivores and antelopes; we have omitted some of the smaller members of the last two groups because we have as yet been unable to obtain sufficiently detailed informa• tion about their distribution. A complete list of the larger Carnivora (i.e. excluding the Mustelidae and Viverridae), the Proboscidea, Perissodactyla and Artiodactyla occurring in Kenya appears at the end of this paper. Those species appearing in brackets have not been dealt with in this paper. The distribution maps are accompanied by four others showing:• (2)(1) altitude;physical features,(3) rainfall;place names,and (4)andvegetation.conservationTheseareas;are drawn from maps in the Atlas of Kenya (1959), with the gratefully acknowledged permission of the Director of Surveys, Kenya. -

Quotas for Leopard Hunting Trophies
Original language: English AC31 Inf. 19 (English only / seulement en anglaise / únicamente en inglés) CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ___________________ Thirty-first meeting of the Animals Committee Online, 31 May, 1, 4, 21 and 22 June 2021 Species specific matters Leopards (Panthera pardus) QUOTAS FOR LEOPARD HUNTING TROPHIES This document has been submitted by the Central African Republic* in relation to agenda item 29.2 on Quotas for leopard hunting trophies. * The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CITES Secretariat (or the United Nations Environment Programme) concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. AC31 Inf. 19 – p. 1 MINISTRY OF WATER, FORESTS, CENTRAL AFRICAN REPUBLIC HUNTING AND FISHING Unity – Dignity – Work ************ CABINET DIRECTOR ************ GENERAL DIRECTORATE OF WATER, FORESTS, HUNTING AND FISHING ************ DEPARTMENT OF WILDLIFE AND Bangui, February 09, 2021 PROTECTED AREAS ************ N° 001/MWFHF/CD/GDWFHF/ DWPA. LEOPARD IN CENTRAL AFRICAN REPUBLIC NON-DETRIMENT FINDINGS By : Nestor WALIWA Director of Wildlife and Protected Areas CITES Management Authority and Focal Point Phone: +236 72278497 / +236 75886711 WhatsApp: +236 72278497 English Version Email: [email protected] Central African Republic TABLE OF CONTENTS 1. HISTORY OF LEOPARD EXPORT QUOTAS IN CENTRAL AFRICAN REPUBLIC .... 3 2. STATUS OF THE LEOPARD IN CENTRAL AFRICAN REPUBLIC ............................... 3 2.1. DISTRIBUTION OF THE LEOPARD IN CENTRAL AFRICAN REPUBLIC ....................................... 3 2.1.1. -

List of Taxa for Which MIL Has Images
LIST OF 27 ORDERS, 163 FAMILIES, 887 GENERA, AND 2064 SPECIES IN MAMMAL IMAGES LIBRARY 31 JULY 2021 AFROSORICIDA (9 genera, 12 species) CHRYSOCHLORIDAE - golden moles 1. Amblysomus hottentotus - Hottentot Golden Mole 2. Chrysospalax villosus - Rough-haired Golden Mole 3. Eremitalpa granti - Grant’s Golden Mole TENRECIDAE - tenrecs 1. Echinops telfairi - Lesser Hedgehog Tenrec 2. Hemicentetes semispinosus - Lowland Streaked Tenrec 3. Microgale cf. longicaudata - Lesser Long-tailed Shrew Tenrec 4. Microgale cowani - Cowan’s Shrew Tenrec 5. Microgale mergulus - Web-footed Tenrec 6. Nesogale cf. talazaci - Talazac’s Shrew Tenrec 7. Nesogale dobsoni - Dobson’s Shrew Tenrec 8. Setifer setosus - Greater Hedgehog Tenrec 9. Tenrec ecaudatus - Tailless Tenrec ARTIODACTYLA (127 genera, 308 species) ANTILOCAPRIDAE - pronghorns Antilocapra americana - Pronghorn BALAENIDAE - bowheads and right whales 1. Balaena mysticetus – Bowhead Whale 2. Eubalaena australis - Southern Right Whale 3. Eubalaena glacialis – North Atlantic Right Whale 4. Eubalaena japonica - North Pacific Right Whale BALAENOPTERIDAE -rorqual whales 1. Balaenoptera acutorostrata – Common Minke Whale 2. Balaenoptera borealis - Sei Whale 3. Balaenoptera brydei – Bryde’s Whale 4. Balaenoptera musculus - Blue Whale 5. Balaenoptera physalus - Fin Whale 6. Balaenoptera ricei - Rice’s Whale 7. Eschrichtius robustus - Gray Whale 8. Megaptera novaeangliae - Humpback Whale BOVIDAE (54 genera) - cattle, sheep, goats, and antelopes 1. Addax nasomaculatus - Addax 2. Aepyceros melampus - Common Impala 3. Aepyceros petersi - Black-faced Impala 4. Alcelaphus caama - Red Hartebeest 5. Alcelaphus cokii - Kongoni (Coke’s Hartebeest) 6. Alcelaphus lelwel - Lelwel Hartebeest 7. Alcelaphus swaynei - Swayne’s Hartebeest 8. Ammelaphus australis - Southern Lesser Kudu 9. Ammelaphus imberbis - Northern Lesser Kudu 10. Ammodorcas clarkei - Dibatag 11. Ammotragus lervia - Aoudad (Barbary Sheep) 12. -
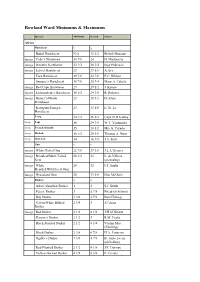
Rowland Ward Minimums & Maximums
Rowland Ward Minimums & Maximums Species Minimum Record Owner Africa Hartebeest x x x Bubal Hartebeest N/A 15 1/2 British Museum image Coke’s Hartebeest 18 7/8 24 D. Mackenzie image Western Hartebeest 22 1/2 28 3/4 Inge Pederson image Lelwel Hartebeest 23 27 5/8 A.Avy Tora Hartebeest 19 7/8 22 7/8 F.C. Hibben Swayne’s Hartebeest 16 7/8 20 3/4 Mary A. Cabela image Red/Cape Hartebeest 23 29 1/2 J. Krause image Lichtenstein’s Hartebeest 18 1/2 24 3/8 R. Rohwer image Hunter’s/Hirola 23 28 1/2 H. Klein Hartebeest Korrigum/Senegal 23 33 1/8 L. St. Lo Hartebeest Tiang 20 1/2 26 1/2 Capt. D.H Gawne image Topi 16 24 3/8 W.T. Yoshimoto image Tsessebe/Sassaby 15 18 1/2 Mrs A. Curado image Blesbok 16 1/2 20 5/8 Thomas A. Hunt image Bontebok 14 16 3/8 J.A. Feist Gnu x x x image White-Tailed Gnu 22 7/8 29 3/8 J.L. L’Ecuyer image Brindled/Black-Tailed 28 1/2 36 L. de Villiers Gnu (picked up) image White 28 32 I.T. Smith Bearded/Wildebeest Gnu image Nyasaland Gnu 28 33 1/8 Don McGuirt Duikers x x x Aders’/Zanzibar Duiker 1 2 S.J. Smith Peters’ Duiker 3 5 7/8 Friedrich Schmitt Bay Duiker 2 3/8 4 7/8 Bela Hidvegi Gabon/White Bellied 2 3/4 5 J.Cousin Duiker image Red Duiker 2 1/2 4 1/8 J.H.M Niblett Harvey’s Duiker 2 1/2 5 R.M. -

Hartebeests in Ethiopia
99 Hartebeests in Ethiopia Melvin Bo/ton In this report on his field survey of Ethiopian hartebeests, two of which—Swayne's, now extinct outside Ethiopia, and the tora—are in the Red Data Book, Melvin Bolton describes the known populations and recommends the appropriate conservation measures. His most encouraging discovery was a population—unfortunately in a heavily cultivated area—of at least 500 Swayne's hartebeest, bringing the estimated total for this subspecies to 600—700. His study shows that it is not always easy to ascribe all hartebeests to the three main subspecies or races, and he describes two intergrades with distinctive features. The field work was helped with small grants from the FPS and the WWF. The hartebeest is still one of the more widespread of large African antelopes although no longer occurring in parts of its former range. If, as some authorities believe, all the forms of A Icelaphus are con- specific then A. buselaphus extends from Senegal in the west to Ethiopia in the east and south to the Cape. In Ethiopia three distinct subspecies or races are found: A. b. lelwel, A. b. tora and A. b. swaynei; the last two are in the IUCN Red Data Book. For convenience in presentation, all Ethiopian hartebeests will be considered under the headings of these three subspecies. Tora Hartebeest A reddish fawn animal with horns which diverge widely outwards from the pedicle then upwards, so that in front view they present a shape often likened to a curly bracket lying on its side, the tora hartebeest occurs in eastern Sudan and north-western Ethiopia. -

Field Report Nubian Giraffe Conservation Assessment in Mwea National Reserve and Ruma National Park, Kenya
FIELD REPORT NUBIAN GIRAFFE CONSERVATION ASSESSMENT IN MWEA NATIONAL RESERVE AND RUMA NATIONAL PARK, KENYA April 2017 Arthur Muneza, Giraffe Conservation Foundation Emmanuel Ngumbi & George Njagi, African Fund for Endangered Wildlife Introduction Giraffe populations are under threat, having declined by almost 40% in the last 30 years (Muller et al. 2016). Among the nine currently recognised subspecies of giraffe (Giraffa camelopardalis), only the Rothschild’s (G. c. rothschildi) and West African (G. c. peralta) giraffe are listed as endangered on the IUCN Red List. These two subspecies have declined significantly over time and lost a majority of their natural habitat, and importantly, remain vastly understudied. More recently, the Giraffe Conservation Foundation (GCF) and partners found that the Rothschild’s giraffe is identical to the Nubian giraffe (G. c. camelopardalis) and as such should be subsumed into it as Nubian giraffe was named first and the nominate species of giraffe (Fennessy et al. 2016). Habitat loss and conversion of land due to pastoralism, agricultural development and human encroachment (Ogutu et al. 2016), combined with illegal hunting, are significant threats to the survival of giraffe throughout their range, in particular East Africa. This highlights a critical need to better understand these two contrasting Nubian giraffe populations and their habitat in Kenya to provide informed conservation management decisions. In November 2016, the GCF and the African Fund for Endangered Wildlife (AFEW) initiated a project to gather new data and update the conservation status of Nubian giraffe in Mwea National Reserve (NR) in central Kenya and Ruma National Park (NP) in western Kenya. Such initiatives are intended to support the implementation of the Kenya Wildlife Service (KWS) draft National Giraffe Conservation Strategy of Kenya, and in the long-term, provide new information for the revision of the Strategy and reassessment of the Nubian giraffe on the IUCN Red list. -

Garamba and Chinko Protected Areas
CENTRAL AFRICA REGIONAL PROGRAM FOR THE ENVIRONMENT (CARPE) - GARAMBA AND CHINKO PROTECTED AREAS OVERVIEW USAID is supporting Garamba National Park (GNP) in northeastern Democratic Republic of the Congo (DRC) and the Chinko Nature Reserve in eastern Central African Republic (CAR), in partnership with African Parks and the governments of DRC and CAR. BACKGROUND GNP is one of the oldest national parks in Africa and covers an area of 4,900 square kilometers, with a buffer zone of hunting reserves to the east, south and west as well as South Sudan’s Lantoto National Park to the north. GNP and the three hunting zones collectively comprise the 14,760 square kilometer Garamba Complex. With both forest and savanna habitats, GNP is home to 165 species of mammals, 363 species of birds, 82 species of reptiles, and 42 species of amphibians. The park lies on the transition zone between two centers of endemism: Guinea-Congolian forest and Guinean-Sudanese savannah, hosting species typical to both of these biogeographic zones. Garamba thus supports important populations of elephant, buffalo and hippo. GNP also contains the last population of Kordofan giraffe in DRC, and is the last known area where the northern white rhino existed in the wild. Large populations of Uganda kob, Lelwel’s hartebeest, buffalo and lion also roam the park. Other important forest species found in the hunting areas include chimpanzees, bongo, giant forest hog and sitatunga. The Chinko Nature Reserve (Chinko), one of the last remaining strongholds for elephants in CAR, covers an area of 19,846 square kilometers. Chinko is situated in the Chinko-Mbari drainage basin in the east of CAR. -

Lf$Fi,F.Y-,.\),I.I..Y SUDAN Oovxeklfesit CATALOGUE OF
lf$fi,f.y-,.\),i.i..y SUDAN OOVXEKlfESiT CATALOGUE OF WILD MAMMALS 07 THB SUDAN occurring in the natural orders ABTIODACTYLA AND PKRI3S0DACTYLA m P.Z. MACKHfZIB M.B.E., M.S.C.V.S. Keeper, Natural History Collection Sudan Museum and Sudan Veterinary Service ^msmmamt SUDAN MDSBUM (HATUHAL BIflTOBY) PUBLICATION No. k PRICB 40 P.T. 1954 SUDAN GOVERNMENT CATALOGUE OP WILD MAMMALS OP THE SUDAN occurring in the natural orders ASTIODACTYLA AND PERISSODACTYLA P.Z. MACKENZIE M. B. E. , M.R.C.V.S. Keeper, Natural History Collection Sudan Museum and Sudan Veterinary Service SUDAN MUSEUM (NATURAL HISTORY) PUBLICATION No. k PRICE kO P.T. 1954 Introduction It is hoped that this short work, which is intended to serve the zoologist, the naturalist and the big game hunter, will toe of some value in identifying the Sudan's larger mammals. The distribution maps and the short note on the relative abundance or otherwise of each species with the aid of descriptive works on African fauna and access to collections of game animal heads, will, it is hoped, help to identify any species seen or collected in the Sudan. The nomenclature of the African fauna has always been a vexed question, for, in the past so many authorities from different countries have collected, identified and named the same species independently and often differently. In this catalogue the latin nomenclature - with three exceptions - is taken entirely from MA Check List of African Mammals" by Glover M. Allen, which appears as Volume LXXXIII, Bulletin of the Museum of Comparative Zoology, Harvard College, U.S.A. -

WHITE-EARED KOB (Kobus Kob Leucotis) in CMS APPENDIX II
CMS Distribution: General CONVENTION ON UNEP/CMS/ScC18/Doc.7.2.12 MIGRATORY 11 June 2014 SPECIES Original: English 18th MEETING OF THE SCIENTIFIC COUNCIL Bonn, Germany, 1-3 July 2014 Agenda Item 7.2 PROPOSAL FOR THE INCLUSION OF THE WHITE-EARED KOB (Kobus kob leucotis) IN CMS APPENDIX II Summary The Government of Ethiopia has submitted a proposal for the inclusion of the White-eared Kob (Kobus kob leucotis) in CMS th Appendix II at the 11 Meeting of the Conference of the Parties (COP11), 4-9 November 2014, Quito, Ecuador. The proposal is reproduced under this cover for its evaluation by th the 18 Meeting of the CMS Scientific Council. For reasons of economy, documents are printed in a limited number, and will not be distributed at the meeting. Delegates are kindly requested to bring their copy to the meeting and not to request additional copies. UNEP/CMS/ScC18/Doc.7.2.12: Proposal II/3 PROPOSAL FOR INCLUSION OF SPECIES ON THE APPENDICES OF THE CONVENTION ON THE CONSERVATION OF MIGRATORY SPECIES OF WILD ANIMALS A. PROPOSAL: Inclusion of White-eared Kob (Kobus kob leucotis) on Appendix II - Migratory species requiring international cooperation B. PROPONENT: Government of Ethiopia C. SUPPORTING STATEMENT 1. Taxon 1.1 Classis Mammalia 1.2 Order Artiodactyla 1.3 Family Bovidae 1.4 Genus/ Species/sub species: Kobus kob leucotis (Lichtenstein and Peters, 1853) 1.5 Common name(s) White-eared Kob 2. Biological data 2.1 Distribution (current and historical) White-eared Kob (Kobus kob leucotis) have the most restricted range of all kob sub species and occurs only in South Sudan, south-west Ethiopia and historically in the extreme north- east Uganda.