Investigating Spatio-Temporal Distribution and Diffusion Patterns Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(I) Kabal BAR ABA KHEL 2 78320
Appointment of Teachers (Adhoc School Based) in Elementary & Secondary Education department, Khyber Pakhutunkhwa (Recruitment Test)) Page No.1 Test held on 20th, 26th & 27th November 2016 Final Merit List (PST-Male) Swat NTS Acad:Ma Marks SSC HSSC Bachelor BS Hons. Master M.Phill Diploma M.Ed/MA.Ed rks [out of 100] [Out of 100] Total (H=A+B+ Candidate RollN Date Of 20% 35% 15% 5% 15% Marks [Out Father Name Total 20% (A) Obt Total 20% (B) Obt Total Obt Total Obt Total Obt Total Obt Total Obt Total 5% (G) C+D+E+ Mobile Union Address REMARKS Tehsil Sr Name School Name Obt (I) of 200] o Birth (C) (C) (D) (E) (F) F+G) Name U.C Name apply for J=H+I Council GPS 78320 0347975 BAR ABA VILLAGE AND POST OFFICE SIR SINAI BAR ABA 2 CHINDAKHW AHMAD ALI 1993-5-8 792.0 1050.015.09 795.0 1100.014.45 0.0 0.0 0.0 3409.04300.027.75 0.0 0.0 0.0 0.0 0.0 0.0 644.0 900.0 10.73 0.0 0.0 0.0 68.02 63.0 131.02 TAHIR ALI 9647 KHEL TEHSIL KABAL SWAT Kabal KHEL 01098 ARA 78320 0347975 BAR ABA VILLAGE AND POST OFFICE SIR SINAI BAR ABA 3 GPS DERO AHMAD ALI 1993-5-8 792.0 1050.015.09 795.0 1100.014.45 0.0 0.0 0.0 3409.04300.027.75 0.0 0.0 0.0 0.0 0.0 0.0 644.0 900.0 10.73 0.0 0.0 0.0 68.02 63.0 131.02 TAHIR ALI 9647 KHEL TEHSIL KABAL SWAT Kabal KHEL 01098 CHUM 78320 0347975 BAR ABA VILLAGE AND POST OFFICE SIR SINAI BAR ABA 3 AHMAD ALI 1993-5-8 792.0 1050.015.09 795.0 1100.014.45 0.0 0.0 0.0 3409.04300.027.75 0.0 0.0 0.0 0.0 0.0 0.0 644.0 900.0 10.73 0.0 0.0 0.0 68.02 63.0 131.02 TAHIR ALI 9647 KHEL TEHSIL KABAL SWAT Kabal KHEL 01098 GPS KABAL 78320 0347975 BAR ABA VILLAGE -

Pdf | 951.36 Kb
P a g e | 1 Operation Updates Report Pakistan: Monsoon Floods DREF n° MDRPK019 GLIDE n° FL-2020-000185-PAK Operation update n° 1; Date of issue: 6/10/2020 Timeframe covered by this update: 10/08/2020 – 07/09/2020 Operation start date: 10/08/2020 Operation timeframe: 6 months; End date: 28/02/2021 Funding requirements (CHF): DREF second allocation amount CHF 339,183 (Initial DREF CHF 259,466 - Total DREF budget CHF 598,649) N° of people being assisted: 96,250 (revised from the initially planned 68,250 people) Red Cross Red Crescent Movement partners currently actively involved in the operation: IFRC Pakistan Country Office is actively involved in the coordination and is supporting Pakistan Red Crescent Society (PRCS) in this operation. In addition, PRCS is maintaining close liaison with other in-country Movement partners: International Committee of the Red Cross (ICRC), German Red Cross (GRC), Norwegian Red Cross (NorCross) and Turkish Red Crescent Society (TRCS) – who are likely to support the National Society’s response. Other partner organizations actively involved in the operation: National Disaster Management Authority (NDMA), Provincial Disaster Management Authorities (PDMAs), District Administration, United Nations (UN) and local NGOs. Summary of major revisions made to emergency plan of action: Another round of continuous heavy rains started in most part of the country on the week of 20 August 2020 until 3 September 2020 intermittently. The second round of torrential rains caused urban flooding in the Sindh province and flash flooding in Khyber Pakhtunkhwa (KP). New areas have been affected by the urban flooding including the districts of Malir, Karachi Central, Karachi West, Karachi East and Korangi (Sindh), and District Shangla, Swat and Charsadda in Khyber Pakhtunkhwa. -

Swat Valley – PML-N – Tehrik-E-Nifaz-E-Shariah- Mohammadi – Landowners – April 2009 Army Offensive – Internal Relocation – Asylum Seekers 23 December 2009
Country Advice Pakistan Pakistan – PAK35880 – Swat Valley – PML-N – Tehrik-e-Nifaz-e-Shariah- Mohammadi – Landowners – April 2009 Army Offensive – Internal relocation – Asylum seekers 23 December 2009 1 Please provide a concise history of recent events in the Swat Valley, including the ‘Talibanisation’ of the valley and the April 2009 offensive by the Pakistan army to retake the valley. 2 What is the law and order situation in the Swat Valley since the April 2009 Pakistan army offensive in the valley? What has become of the Taliban leadership and militants in the valley since the offensive? Do they still pose a threat to the peace and stability of the valley? Origins of the Tehreek Nifaz e Sharia Mohammadi in the Swat Valley The terms ‘Taliban’ and ‘Taliban in Pakistan’ (TIP) are often employed by the Pakistani and International media as shorthand for a number of militant Islamic groups espousing the introduction of Sharia law, the creation of an Islamic state or the pursuit of violent jihad. It has been reported that a number of such groups currently operate in the Malakand district of North West Frontier Province, which includes the Swat Valley. In the Swat region itself, the primary movement since the early 1990s to the present day has been the Tehrik-e-Nifaz-e- Shariah-Mohammadi (TNSM). The February 2009 IRIN article ‘Pakistan: Origins of the violence in Swat Valley’ provides useful and concise information on the Tehrik-e-Nifaz-e-Shariah-Mohammadi (TNSM). On the subject of its origins IRIN states “[i]n 1992, Sufi Mohammad Khan established the Tehrik- e-Nifaz-e-Shariah-Mohammadi (TNSM) in Swat, as a party seeking an Islamic order. -

Socio-Economic Conditions of Post-Conflict Swat: a Critical Appraisal
TIGAH,,, A JOURNAL OF PEACE AND DEVELOPMENT Volume: II, December 2012, TigahFATA Research Centre, Islamabad Socio-Economic Conditions of Post-Conflict Swat: A Critical Appraisal * Dr. Salman Bangash Background of Conflict in Swat The Pakistani province of Khyber Pakhtunkhwa (KPK), previously known as the North West Frontier Province (NWFP) lies between the Indus River in the east and the Suleiman mountain range in the west, with an area of 74,521 sq. km. It comprises of 18 districts and Provincially Administered Tribal Area (PATA), consisting of Malakand Agency, which is further divided into districts of Upper Dir, Lower Dir, Chitral, Swat, Buner, Shangla and areas of Kala Dhaka. Swat is one of the districts of PATA, Malakand Division. Swat is a mountainous region with varying elevations, ranging from 600 meters to 6000 meters above the sea level, from south to north to the foothills of Hindukush mountain range. The region is blessed with abundance of water in shape of the Swat River. It also has forests, lush green valleys, plains and glaciers. The Swat valley is rich in flora and fauna. It is famous for its variety of fruits, medicinal herbs and botanical plants. The total area of District Swat is 5337 sq. km, divided into two tehsils, namely Matta (683 sq. km) and Swat (4654 sq. km).The total forest cover in Swat is 497,969 acres which consists of varieties of Pine trees. The District Headquarter of Swat is Saidu Sharif, but the main town in the district is Mingora. Saidu Sharif is at a distance of 131 km from Peshawar, the provincial capital, towards the northeast, * The author is a Lecturer at the Department of History, University of Peshawar. -

DETAILS of Npos, SOCIAL WELFARE DEPARTMENT KHYBER PAKHTUNKHWA (Final Copy)
DETAILS OF NPOs, SOCIAL WELFARE DEPARTMENT KHYBER PAKHTUNKHWA (Final copy) (i) (ii) (iii) (iv) (v) (vi) (vii) (viii) (ix) (x) (xi) (xii) (xiii) (xiv) (xv) (xvi) (xvii) Name, Address & Contact No. Registration No. Sectors/ Target Size Latest Key Functionaries Persons in Effective Name & Value of Associate Bank Donor Means Mode Cross- Recruitme Detail of of NPO with Registering Function Area and Audited Control Moveable & d Entities Account Base of of Fund border nt Criminal Authority s Communit Accounts Immovable (if any) Details Paymen Payme Activiti Capabilitie /Administrati y available Assets (Bank, t nt es s ve Action (Yes /No) Branch & against NPO Account No.) (if any) 1 AAGHOSH WELFARE DSW/NWFP/254 Educatio Peshawar Mediu Yes Education Naseer Ahmad 01 Lack No;. Nil No. NA N.A N.A 07 Nil ORGANIZATION , ISLAMIA 9 n and m 03009399085 PUBLIC SCHOOL 09-03-2006 General aaghosh_2549@yahoo. BHATYAN CHARSADA Welfare com.com ROAD PESHAWAR 2 ABASEEN FOUNDATION DSW/NWFP/169 Educatio Peshawar mediu 2018 Education Dr. Mukhtiar Zaman 80 lac Nil --------- Both Bank Chequ Nil 20 Nil PAK, 3rd Floor, 272 Deans 9 n & m Tel: 0092 91 5603064 e Trade Centre, Peshawar 09.09.2000 health [email protected] Cantonment, Peshawar, . KPK, Pakistan. 3 Ahbab Welfare Organization, DSW/KPK/3490 Health Peshwar Small 2018 Dr. Habib Ullah 06 lac Nil ---------- Self Cash Cash Nil 08 Nil Sikandarpura G.t Rd 16.03.2011 educatio 0334-9099199 help Cheque Chequ n e 4 AIMS PAKISTAN DSW/NWFP/228 Patient’s KPK Mediu 2018 Patient’s Dr. Zia ul hasan 50 Lacs Nil 1721001193 Local Throug Bank Nil Nil 6-A B-3 OPP:Edhi home 9 Diabetic m Diabetic Welfare 0332 5892728, 690001 h Phase #05 Hayatabad 24,03.04 Welfare /Awareness 091-5892728 MIB Cheque Peshawar. -

Revision of Election Electoral Rolls
Changes involved (if DISTRICT TEHSIL QH PC VILLAGE CRCODE NAME DESG PHONE ADDRESS any) i.e. Retirement, Transfer etc 1 2 3 4 5 6 7 8 9 10 11 SWAT BABUZAI BABUZAI QH QAMBAR PC 0070101 ANWAR ALI SST 03025740801 GHS GOGDARA SWAT BABUZAI BABUZAI QH MINGORA PC 0070102 HAZRAT HUSSAIN CT 03349321527 GHS NO,4 MINGORA SWAT BABUZAI BABUZAI QH SAIDU SHARIF PC 0070103 MUZAFAR HUSSAIN SCT 03449895384 GHS BANR MINGORA SWAT BABUZAI BABUZAI QH MARGHAZAR PC 0070104 SHAMROZ KHAN SST,3 03345652060 GHS CHITOR SWAT BABUZAI BABUZAI QH JAMBIL PC 0070105 ANWAR ULLAH SST 03429209704 GHS KOKARAI SWAT BABUZAI BABUZAI QH KOKARAI PC 0070106 MINHAJ PSHT 03149707774 GPS KOKARAI SWAT BABUZAI BABUZAI QH MANGLAWAR PC 0070107 SAID AKRAM SHAH NULL 03459526902 GPS TOTKAI SWAT BABUZAI BABUZAI QH BISHBANR PC 0070108 ABDUL QAYUM PSHT 03459522939 GPS WARA SAR SWAT BABUZAI BABUZAI QH SARSARDARAY PC 0070109 M. KHALIQ PSHT 03449892194 GPS DIWAN BAT SWAT BABUZAI BABUZAI QH ODIGRAM PC 0070110 ASGHAR KHAN PET 03469411106 GHS TINDODOG SWAT BABUZAI BABUZAI QH ODIGRAM PC 0070110 PARVANAT KHAN HM 03450384634 GHS GOGDARA SWAT BABUZAI MINGORA M.C. CHARGE NO 02 CIRCLE NO 01 0070201 SHER AFZAL KHAN SST NULL GHS NO.1 SWAT BABUZAI MINGORA M.C. CHARGE NO 02 CIRCLE NO 02 0070202 AMIR MOHAMMAD SCT NULL GHSS HAJI BABA SWAT BABUZAI MINGORA M.C. CHARGE NO 02 CIRCLE NO 03 0070203 ZAHID KHAN SCT NULL GHSS HAJI BABA SWAT BABUZAI MINGORA M.C. CHARGE NO 02 CIRCLE NO 04 0070204 MUHAMMAD RAHIM SST NULL GHS NO.1 MINGORA SWAT BABUZAI MINGORA M.C. -

Communicating-Change
COMMUNICATING A collection of successful local government initiatives under municipal CHANGE service delivery in Malakand Local Government, Elections and Rural Development Department Government of Khyber Pakhtunkhwa Developed with the German technical cooperation of the Support to Good Governance in Pakistan Programme Content, layout and photography: DOT Advertising All rights are reserved by GIZ. No part of this book may be reproduced by any means without written permission. Reproduction for non-commercial purposes is permitted provided the source is named. COMMUNICATING A collection of successful local government initiatives under municipal CHANGE service delivery in Malakand Deutsche Gesellschaft fur Internationale Zusammenarbeit (GIZ) GmbH COMMUNICATING CHANGE A collection of successful local government initiatives under municipal service delivery in Malakand We measure our success not by the number of projects completed but by the positive change these projects bring in the lives of citizens. The restructuring of the local government system through the Khyber Pakhtunkhwa Local Government Act 2013 is another major step forward in our efforts to make public goods and services available and accessible to everyone without any exception. We believe effective local governments work for the people and reflect their needs as closely as possible - and that is where communication is positioned to play a key role. For us, communication is an important medium to inform you about our priorities and achievements. At the same time, it goes far beyond that. We are strengthening two- way communication mechanisms to foster responsive local governments and informed citizens. Both are crucial for a meaningful dialogue. The five municipalities of Adenzai, Bahrain, Barikot, Kabal and Khwazakhela were established in 2010 as a result of re-configuration of the administrative setup in Malakand Division to address security measures and flood damages. -

(Morels) of Utror-Gabral Valleys, District Swat, Pakistan by Muhammad Hamayun¹ and Mir Ajab Khan² ¹ Lecturer, Govt
Studies on collection and marketing of Morchella (Morels) of Utror-Gabral Valleys, District Swat, Pakistan By Muhammad Hamayun¹ and Mir Ajab Khan² ¹ Lecturer, Govt. Degree College, Swabi, Pakistan ² Associate Professor, Department of Biological Sciences, Quaid-e-Azam University, Islamabad, Pakistan Address for Correspondence: Muhammad Hamayun, Lecturer in Botany, Govt. Degree College Kotha (Swabi), NWFP, Pakistan E.mail, M.Hamayun: [email protected] Abstract This paper is based on a research project carried out to study the collection and marketing status of morels in the remote HinduKush-Himalayan regions of Utror and Gabral, Pakistan. Eight species of morels were found to be collected in the project area during the months of March to July. Morchella conica and Morchella esculenta were the major species collected in the area. These morels are sold in the local markets of Madyan and Mingora, from where they are exported to France, Belgium, Switzerland, Austria and Germany. Morels thus provide a vital source of income to the poor population of Utror and Gabral. Morel collectors include 38.0% women, 37.0% men and 25.0% children. Huge quantities of morels are lost each year due to improper storage and collection techniques. Key words: Morels; Marketing; Hindukush-Himalayas; Commerce Introduction of Study Site The Utror-Gabral valleys are situated in the north western part of the District Swat, Pakistan. The project area has unique flora as it occupies nexus of the three great mountain ranges i.e. Himalayas, Hindukush and Karakoram. The altitude of Utror is 2,225 meters and Gabral is 2,550 meters from the sea level. -

Forestry in the Princely State of Swat and Kalam (North-West Pakistan)
Forestry in the Princely State of Swat and Kalam (North-West Pakistan) A Historical Perspective on Norms and Practices IP6 Working Paper No.6 Sultan-i-Rome, Ph.D. 2005 Forestry in the Princely State of Swat and Kalam (North-West Pakistan) A Historical Perspective on Norms and Practices IP6 Working Paper No.6 Sultan-i-Rome, Ph.D. 2005 The Swiss National Centre of Competence in Research (NCCR) North-South is based on a network of partnerships with research institutions in the South and East, focusing on the analysis and mitigation of syndromes of global change and globalisation. Its sub-group named IP6 focuses on institutional change and livelihood strategies: State policies as well as other regional and international institutions – which are exposed to and embedded in national economies and processes of globalisation and global change – have an impact on local people's livelihood practices and strategies as well as on institutions developed by the people themselves. On the other hand, these institutionally shaped livelihood activities have an impact on livelihood outcomes and the sustainability of resource use. Understanding how the micro- and macro-levels of this institutional context interact is of vital importance for developing sustainable local natural resource management as well as supporting local livelihoods. For an update of IP6 activities see http://www.nccr-north-south.unibe.ch (>Individual Projects > IP6) The IP6 Working Paper Series presents preliminary research emerging from IP6 for discussion and critical comment. Author Sultan-i-Rome, Ph.D. Village & Post Office Hazara, Tahsil Kabal, Swat–19201, Pakistan e-mail: [email protected] Distribution A Downloadable pdf version is availale at www.nccr- north-south.unibe.ch (-> publications) Cover Photo The Swat Valley with Mingawara, and Upper Swat in the background (photo Urs Geiser) All rights reserved with the author. -

HEALTH CLUSTER PAKISTAN Crisis in Khyber Pakhtunkhwa
HEALTH CLUSTER PAKISTAN Crisis in Khyber Pakhtunkhwa Issue No 5 12 April ‐ 5 May 2010 Highlights • As of 6th May, 2010, 341,768 people (approximately 48,824 families) from Orakzai and Kurram Agency, of Khyber Pakhtunkhwa have become IDPs and are living in host communities. Of the total IDPs, 111,482 people (approx. 15,926 families) are living in Hungu, 27,013 people (approx. 3,859 families) in Peshawar and 203,273 people (approx. 29,039 families) in Kohat.. • There are another 1,513,713 IDP’s (approx207,358 families) residing in host communities of Mardan, Peshawar, Swabi, Charsadda, Nowshera, Kohat, Hangu, Tank, DI-Khan, Abbotabad, Haripur, Mansehra and Battagram districts of Khyber Pakhtunkhwa. Besides the IDPs living in host communities, 124,169 IDPs (approx 24,626 families) are living in camps. Jalozai is the largest camp with a population of 111,798 people (22,532 families). (Multiple Sources: CAR reports, SSG, PDMA PaRRSA, NADRA, Social Welfare Department) • Health Directorate, FATA, WHO and UNICEF have conducted assessment of Health facilities in Mohmand agency in preparation for the expected return of IDPs from Jalozai camp. • 517 DEWS health facilities reported a total of 317,084 consultations carried out from 17th April, 2010 to 30th April, 2010. 184,374 (58 %) of the total consultations were reported for female patients while 132,710 (42%) consultations were carried out for male patients. Children aged under 5 years represented 78,742 (25%) of all consultations. 72 health facilities reported 2,675 visits for antenatal care. • For the period 17 April to 30 April, Twenty four alerts – one alert for acute flaccid paralysis (AFP), fifteen alerts of suspected measles, and two alerts of Acute Watery Diarrhoea (AWD), were received from Tank, D.I Khan, Lower Dir, Mardan, Nowshera, Peshawar, Swabi and Swat. -
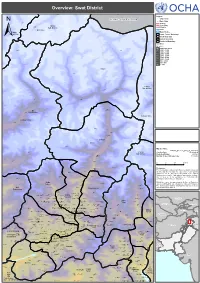
Swat District !
! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Overview: Swat District ! ! ! ! SerkiSerki Chikard Legend ! J A M M U A N D K A S H M I R Citiy / Town ! Main Cities Lohigal Ghari ! Tertiary Secondary Goki Goki Mastuj Shahi!Shahi Sub-division Primary CHITRAL River Chitral Water Bodies Sub-division Union Council Boundary ± Tehsil Boundary District Boundary ! Provincial Boundary Elevation ! In meters ! ! 5,000 and above Paspat !Paspat Kalam 4,000 - 5,000 3,000 - 4,000 ! ! 2,500 - 3,000 ! 2,000 - 2,500 1,500 - 2,000 1,000 - 1,500 800 - 1,000 600 - 800 0 - 600 Kalam ! ! Utror ! ! Dassu Kalam Ushu Sub-division ! Usho ! Kalam Tal ! Utrot!Utrot ! Lamutai Lamutai ! Peshmal!Harianai Dir HarianaiPashmal Kalkot ! ! Sub-division ! KOHISTAN ! ! UPPER DIR ! Biar!Biar ! Balakot Mankial ! Chodgram !Chodgram ! ! Bahrain Mankyal ! ! ! SWAT ! Bahrain ! ! Map Doc Name: PAK078_Overview_Swat_a0_14012010 Jabai ! Pattan Creation Date: 14 Jan 2010 ! ! Sub-division Projection/Datum: Baranial WGS84 !Bahrain BahrainBarania Nominal Scale at A0 paper size: 1:135,000 Ushiri ! Ushiri Madyan ! 0 5 10 15 kms ! ! ! Beshigram Churrai Churarai! Disclaimers: Charri The designations employed and the presentation of material Tirat Sakhra on this map do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Beha ! Nations concerning the legal status of any country, territory, Bar Thana Darmai Fatehpur city or area or of its authorities, or concerning the Kwana !Kwana delimitation of its frontiers or boundaries. Kalakot Matta ! Dotted line represents a!pproximately the Line of Control in Miandam Jammu and Kashmir agreed upon by India and Pakistan. Sebujni Patai Olandar Paiti! Olandai! The final status of Jammu and Kashmir has not yet been Gowalairaj Asharay ! Wari Bilkanai agreed upon by the parties. -

Final List of Associate Members for Election 2021-22
FINAL LIST OF ASSOCIATE MEMBERS FOR ELECTION 2021-22 Firm Name Firm Address Representative Mob# CNIC No 1. ROCKCITY RESORT HOTEL KALAM ROAD FIZAGHAT SWAT MUHAMMAD AYUB 923005745313 15602-3910398-5 KHAN 2. MOON LIGHT SILK MILLS G.T RAOD, AMANKOT , MINGORA SWAT GOHAR AYUB KHAN 15602-4109193-7 3. RADO WATCH COMPANY NEW ROAD , MOHALLA ESSA KHEL , AKHTAR MUNIR 923439606621 1560-0485408-7 MINGORA SWAT 4. FAZAL GEM STONE DEALER MADYAN ROAD , MINGORA SWAT FAZAL NAWAB 923469474004 15602-0433664-7 5. SAMI TRADERS SAMI PLAZA , POST OFFICE ROAD, SAMIULLAH 923005741920 15602-6746151-9 MINGORA SWAT 6. RAHMAN AUTO MOBILE AZIZ ABAD NEAR ZAMARUD KAN MINGORA MUHAMMAD RAHMAN 923004247303 15602-0451523-5 SWAT 7. SILK ROAD COLLECTION G.T ROAD ODIGRAM SWAT ISHTIAQ AHAMD 03469462342 15602-2492392-5 8. S.G.B TRADERS G.T ROAD AKA KHAIL MARKET MINGORA USMAN ALI 923018522416 42401-7196035-8 SWAT 9. FAFA GROUP FAZAL SOAP AND MALKIDAM BARIKOT SWAT FAZAL MUHAMMAD 923002140474 15602-0374803-9 WASHING POWDER FACTORY 10. KHUSHAL GEM STONE MAIN BAZAR MINGORA SWAT ZARIN GUL 923339486323 15602-8704582-9 11. ASIAN GALLERY EXPORTER KOTMERA MADYAN ROAD, CHARBAGH JUMA KHAN 923339119813 15602-0355875-1 HANDICRAFTS SWAT 12. KOHSAR SILK INDUSTRY HEAD OFFICE RASHID ABAD KHALID KHAN 923459516620 15602-2312788-7 INGARODHERAI SWAT 13. ITTEFAQ TRADING COMPANY G.T ROAD, AMANKOT DISTT: SWAT BAKHTIAR ALI 923339483680 15602-0445507-3 14. UNITECH CHEMICALS G.T ROAD RAHIMA ABAD MINGORA SWAT ISRAR UL HAQ 923009074191 15602-3503103-5 INDUSTRIES 15. HOTEL ALHARAMIAN AND OPP GENERAL BUS STAND, G.T ROAD ZAHID KHAN 923469406789 15602-7427410-7 RESTURENT MINGORA SWAT 16.