The Molecular Basis of Variation in Human Color Vision
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Introduction to the Chemistry of Dyes
GENERAL INTRODUCTION TO THE CHEMISTRY OF DYES 1. Principles of Colour Chemistry 1.1 Basis for colour Unlike most organic compounds, dyes possess colour because they 1) absorb light in the visible spectrum (400–700 nm), 2) have at least one chromophore (colour-bearing group), 3) have a conjugated system, i.e. a structure with alternating double and single bonds, and 4) exhibit resonance of electrons, which is a stabilizing force in organic compounds (Abrahart, 1977). When any one of these features is lacking from the molecular structure the colour is lost. In addition to chromophores, most dyes also contain groups known as auxochromes (colour helpers), examples of which are carboxylic acid, sulfonic acid, amino, and hydroxyl groups. While these are not responsible for colour, their presence can shift the colour of a colourant and they are most often used to influence dye solubility. Figure 1 shows the relationships between wavelength of visible and colour absorbed/observed. Other factors contributing to colour are illustrated in Figures 2–4. Fig. 1. Wavelength of light absorption versus colour in organic dyes Wavelength Absorbed (nm) Colour Absorbed Colour Observed 400–435 Violet Yellow-Green 435–480 Blue Yellow 480–490 Green-Blue Orange 490–500 Blue-Green Red 500–560 Green Purple 560–580 Yellow-Green Violet 580–595 Yellow Blue 595–605 Orange Green-Blue 605–700 Red Blue-Green –55– 56 IARC MONOGRAPHS VOLUME 99 Fig. 2. Examples of chromophoric groups present in organic dyes O O N N N N O N N O H N Anthraquinone H N Nitro Azo N N Ar C N N C Ar Methine Phthalocyanine Triarylmethane Fig. -

Blue Cone Monochromacy: Visual Function and Efficacy Outcome Measures for Clinical Trials
RESEARCH ARTICLE Blue Cone Monochromacy: Visual Function and Efficacy Outcome Measures for Clinical Trials Xunda Luo1☯‡, Artur V. Cideciyan1☯‡*, Alessandro Iannaccone2, Alejandro J. Roman1, Lauren C. Ditta2, Barbara J. Jennings2, Svetlana A. Yatsenko3, Rebecca Sheplock1, Alexander Sumaroka1, Malgorzata Swider1, Sharon B. Schwartz1, Bernd Wissinger4, Susanne Kohl4, Samuel G. Jacobson1* 1 Scheie Eye Institute, Department of Ophthalmology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, United States of America, 2 Hamilton Eye Institute, Department of Ophthalmology, University of Tennessee Health Science Center, Memphis, Tennessee, United States of America, 3 Pittsburgh Cytogenetics Laboratory, Center for Medical Genetics and Genomics, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania, United States of America, 4 Molecular Genetics Laboratory, Institute for Ophthalmic Research, Centre for Ophthalmology, University of Tuebingen, Tuebingen, Germany ☯ These authors contributed equally to this work. ‡ OPEN ACCESS These authors are joint first authors on this work. * [email protected] (SGJ); [email protected] (AVC) Citation: Luo X, Cideciyan AV, Iannaccone A, Roman AJ, Ditta LC, Jennings BJ, et al. (2015) Blue Cone Monochromacy: Visual Function and Efficacy Abstract Outcome Measures for Clinical Trials. PLoS ONE 10(4): e0125700. doi:10.1371/journal.pone.0125700 Academic Editor: Dror Sharon, Hadassah-Hebrew University Medical Center, ISRAEL Background Blue Cone Monochromacy (BCM) is an X-linked retinopathy caused by mutations in the Received: December 29, 2014 OPN1LW / OPN1MW gene cluster, encoding long (L)- and middle (M)-wavelength sensitive Accepted: March 21, 2015 cone opsins. Recent evidence shows sufficient structural integrity of cone photoreceptors in Published: April 24, 2015 BCM to warrant consideration of a gene therapy approach to the disease. -

Photosynthesis
20 Photosynthesis Plants use sunlight (Photon), (H O) and (CO ) to convert light Energy into chemical Energy by 2 2 chlorophyll. This process is known as photosynthesis. Chemical equation : Sunlight 6CO + 12H O o C H O + 6O + 6H O 2 2 Chlorophyll 6 12 6 2 2 Necessary factors for photoynthesis : Light Chlorophyll Water (H O) 2 Carbon Dioxide (CO ) (0.03 % in the atmosphere) 2 Photosynthetic Organisms : Different types of algae (Blue green algae, Brown algae, Red algae, Green algae). Bryophytes, Pteridophytes, Gymnosperms, Angiosperms. Some bacteria. (Note : Fungi is not able to perform photosynthesis due to lack of chlorophyll.) Advantages of Photosynthesis : Main products of photosynthesis are starch and sucrose. (O ) is byproduct which is used by organisms for respiration. 2 (1) What is produced as byproduct of photosynthesis ? (A) Oxygen (B) Nitrogen (C) Carbon dioxide (D) Sulphur dioxide (2) Which type of energy can be used by all organisms ? (A) Light energy (B) Chemical energy (C) Heat energy (D) Water potential (3) Which of the following type of reaction photosynthesis is ? (A) Anabolic, Endothermic, Reduction (B) Anabolic, Endothermic, Oxidation (C) Catabolic, Exothermic, Oxidation (D) Catabolic, Endothermic, Reduction Answers : (1-A), (2-B), (3-A) 382 History of Photosynthesis : No. Name of Scientist Contribution 1. Joseph Priestly Plants obtain CO from atmosphere and release O . 2 2 (1733-1804) 2. Ingenhouse In bright sunlight, small bubbles were formed around the (1730-1799) green parts of the plant. 3. Julius Von Sachs Green substance (chlorophyll) in plants is located in special (1854) bodies (chloroplast) of plant cell. This green substances produces glucose which is usually stored in the form of starch. -

The Genetics of Normal and Defective Color Vision
Vision Research xxx (2011) xxx–xxx Contents lists available at ScienceDirect Vision Research journal homepage: www.elsevier.com/locate/visres Review The genetics of normal and defective color vision Jay Neitz ⇑, Maureen Neitz University of Washington, Dept. of Ophthalmology, Seattle, WA 98195, United States article info a b s t r a c t Article history: The contributions of genetics research to the science of normal and defective color vision over the previ- Received 3 July 2010 ous few decades are reviewed emphasizing the developments in the 25 years since the last anniversary Received in revised form 25 November 2010 issue of Vision Research. Understanding of the biology underlying color vision has been vaulted forward Available online xxxx through the application of the tools of molecular genetics. For all their complexity, the biological pro- cesses responsible for color vision are more accessible than for many other neural systems. This is partly Keywords: because of the wealth of genetic variations that affect color perception, both within and across species, Color vision and because components of the color vision system lend themselves to genetic manipulation. Mutations Cone photoreceptor and rearrangements in the genes encoding the long, middle, and short wavelength sensitive cone pig- Colorblindness Cone mosaic ments are responsible for color vision deficiencies and mutations have been identified that affect the Opsin genes number of cone types, the absorption spectra of the pigments, the functionality and viability of the cones, Evolution and the topography of the cone mosaic. The addition of an opsin gene, as occurred in the evolution of pri- Comparative color vision mate color vision, and has been done in experimental animals can produce expanded color vision capac- Cone photopigments ities and this has provided insight into the underlying neural circuitry. -

Identification and Distribution of Dietary Precursors of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Vision Research 39 (1999) 219–229 Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC David R. Giovannucci *, Robert S. Stephenson Department of Biological Sciences, Wayne State Uni6ersity, Detroit, MI 48202, USA Received 3 July 1997; received in revised form 10 November 1997 Abstract A dietary source of retinoid or carotenoid has been shown to be necessary for the biosynthesis of functional visual pigment in flies. In the present study, the larvae or adults of Drosophila melanogaster were administered specific carotenoid-containing diets and high performance liquid chromatography was used to identify and quantify the carotenoids in extracts of wild type and ninaD visual mutant flies. When b-carotene was fed to larvae, wild type flies were shown to hydroxylate this molecule and to accumulate zeaxanthin and a small amount of b-cryptoxanthin. Zeaxanthin content was found to increase throughout development and was a major carotenoid peak detected in the adult fly. Carotenoids were twice as effective at mediating zeaxanthin accumulation when provided to larvae versus adults. In the ninaD mutant, zeaxanthin content was shown to be specifically and significantly altered compared to wild type, and was ineffective at mediating visual pigment synthesis when provided to both larval and adult mutant flies. It is proposed that zeaxanthin is the larval storage form for subsequent visual pigment chromophore biosynthesis during pupation, that zeaxanthin or b-crytoxanthin is the immediate precursor for light-independent chromophore synthesis in the adult, and that the ninaD mutant is defective in this pathway. -

Vision Science Meets Visualization
Vision Science Meets Visualization Organizers: Christine Nothelfer Zoya Bylinskii Madison Elliott Cindy Xiong Danielle Albers Szafir Northwestern Massachusetts Institute University of British Northwestern University of Colorado University of Technology Columbia University Boulder Panelists: Ronald Rensink Todd Horowitz Steven Franconeri Karen Schloss Ruth Rosenholtz University of British National Cancer Northwestern University of Massachusetts Institute Columbia Institute University Wisconsin-Madison of Technology presenters each year. Recent developments in these research ABSTRACT topics (e.g., in visual attention, scene understanding, and quantity Vision science can explain what people see when looking at a perception) can inform our understanding of how people interpret visualization--what data features people attend to, what statistics visualized information. However, historically, few VIS they ascertain, and what they ultimately remember. These findings researchers have attended VSS, and few vision scientists have have significant relevance to visualization and can guide effective attended IEEE VIS. This limited overlap has stifled the exchange techniques and design practices. Intersections between of information, ideas, and questions between the two visualization and vision science have traditionally built upon communities. topics such as visual search, color perception, and pop-out. However, there is a broader space of vision science concepts that Further, while crossover between vision science and visualization could inform and explain ideas in visualization but no dedicated is not without precedent (e.g., work by Cleveland & McGill [2] venue for collaborative exchanges between the two communities. and Healey & Enns [8]), these interactions can benefit from This panel provides a space for this exchange by bringing five exposure to a broader set of vision science topics, such as object vision science experts to IEEE VIS to survey the modern vision tracking, ensemble statistics, and visual crowding. -

Colour Vision Deficiency
Eye (2010) 24, 747–755 & 2010 Macmillan Publishers Limited All rights reserved 0950-222X/10 $32.00 www.nature.com/eye Colour vision MP Simunovic REVIEW deficiency Abstract effective "treatment" of colour vision deficiency: whilst it has been suggested that tinted lenses Colour vision deficiency is one of the could offer a means of enabling those with commonest disorders of vision and can be colour vision deficiency to make spectral divided into congenital and acquired forms. discriminations that would normally elude Congenital colour vision deficiency affects as them, clinical trials of such lenses have been many as 8% of males and 0.5% of femalesFthe largely disappointing. Recent developments in difference in prevalence reflects the fact that molecular genetics have enabled us to not only the commonest forms of congenital colour understand more completely the genetic basis of vision deficiency are inherited in an X-linked colour vision deficiency, they have opened the recessive manner. Until relatively recently, our possibility of gene therapy. The application of understanding of the pathophysiological basis gene therapy to animal models of colour vision of colour vision deficiency largely rested on deficiency has shown dramatic results; behavioural data; however, modern molecular furthermore, it has provided interesting insights genetic techniques have helped to elucidate its into the plasticity of the visual system with mechanisms. respect to extracting information about the The current management of congenital spectral composition of the visual scene. colour vision deficiency lies chiefly in appropriate counselling (including career counselling). Although visual aids may Materials and methods be of benefit to those with colour vision deficiency when performing certain tasks, the This article was prepared by performing a evidence suggests that they do not enable primary search of Pubmed for articles on wearers to obtain normal colour ‘colo(u)r vision deficiency’ and ‘colo(u)r discrimination. -

Ba3(P1−Xmnxo4)2 : Blue/Green Inorganic Materials Based on Tetrahedral Mn(V)
Bull. Mater. Sci., Vol. 34, No. 6, October 2011, pp. 1257–1262. c Indian Academy of Sciences. Ba3(P1−xMnxO4)2 : Blue/green inorganic materials based on tetrahedral Mn(V) SOURAV LAHA, ROHIT SHARMA, S V BHAT†, MLPREDDY‡, J GOPALAKRISHNAN∗ and S NATARAJAN Solid State and Structural Chemistry Unit, †Department of Physics, Indian Institute of Science, Bangalore 560 012, India ‡Chemical Science and Technology Division, National Institute for Interdisciplinary Science and Technology (NIIST), Thiruvananthapuram 695 019, India MS received 11 May 2011 ) 3− 2 Abstract. We describe a blue/green inorganic material, Ba3(P1−xMnxO4 2 (I) based on tetrahedral MnO4 :3d chromophore. The solid solutions (I) which are sky-blue and turquoise-blue for x ≤ 0·25 and dark green for x ≥ 0·50, 3− are readily synthesized in air from commonly available starting materials, stabilizing the MnO4 chromophore in an isostructural phosphate host. We suggest that the covalency/ionicity of P–O/Mn–O bonds in the solid solutions tunes the crystal field strength around Mn(V) such that a blue colour results for materials with small values of x. The material could serve as a nontoxic blue/green inorganic pigment. Keywords. Blue/green inorganic material; barium phosphate/manganate(V); tetrahedral manganate(V); blue/green chromophore; ligand field tuning of colour. 1. Introduction field transitions within an unusual five coordinated trigo- nal bipyramidal Mn(III). A turquoise-blue solid based on Inorganic solids displaying bright colours are important Li1·33Ti1·66O4 spinel oxide wherein the colour arises from as pigment materials which find a wide range of appli- an intervalence charge-transfer between Ti3+ and Ti4+ has cations in paints, inks, plastics, rubbers, ceramics, ena- also been described recently (Fernández-Osorio et al 2011). -

1468.Full.Pdf
1468 • The Journal of Neuroscience, February 11, 2004 • 24(6):1468–1477 Cellular/Molecular Novel Features of Cryptochrome-Mediated Photoreception in the Brain Circadian Clock of Drosophila Andre´ Klarsfeld, Se´bastien Malpel, Christine Michard-Vanhe´e, Marie Picot, Elisabeth Che´lot, and Franc¸ois Rouyer Institut de Neurobiologie Alfred Fessard, Centre National de la Recherche Scientifique, Unite´ Propre de Recherche 2216, 91198 Gif-sur-Yvette, France In Drosophila, light affects circadian behavioral rhythms via at least two distinct mechanisms. One of them relies on the visual photo- transduction cascade. The other involves a presumptive photopigment, cryptochrome (cry), expressed in lateral brain neurons that control behavioral rhythms. We show here that cry is expressed in most, if not all, larval and adult neuronal groups expressing the PERIOD (PER) protein, with the notable exception of larval dorsal neurons (DN2s) in which PER cycles in antiphase to all other known cells. Forcing cry expression in the larval DN2s gave them a normal phase of PER cycling, indicating that their unique antiphase rhythm is related to their lack of cry expression. We were able to directly monitor CRY protein in Drosophila brains in situ. It appeared highly unstable in the light, whereas in the dark, it accumulated in both the nucleus and the cytoplasm, including some neuritic projections. We also show that dorsal PER-expressing brain neurons, the adult DN1s, are the only brain neurons to coexpress the CRY protein and the photoreceptor differentiation factor GLASS. Studies of various visual system mutants and their combination with the cryb mutation indicated that the adult DN1s contribute significantly to the light sensitivity of the clock controlling activity rhythms, and that this contribution depends on CRY. -

1 Human Color Vision
CAMC01 9/30/04 3:13 PM Page 1 1 Human Color Vision Color appearance models aim to extend basic colorimetry to the level of speci- fying the perceived color of stimuli in a wide variety of viewing conditions. To fully appreciate the formulation, implementation, and application of color appearance models, several fundamental topics in color science must first be understood. These are the topics of the first few chapters of this book. Since color appearance represents several of the dimensions of our visual experience, any system designed to predict correlates to these experiences must be based, to some degree, on the form and function of the human visual system. All of the color appearance models described in this book are derived with human visual function in mind. It becomes much simpler to understand the formulations of the various models if the basic anatomy, physiology, and performance of the visual system is understood. Thus, this book begins with a treatment of the human visual system. As necessitated by the limited scope available in a single chapter, this treatment of the visual system is an overview of the topics most important for an appreciation of color appearance modeling. The field of vision science is immense and fascinating. Readers are encouraged to explore the liter- ature and the many useful texts on human vision in order to gain further insight and details. Of particular note are the review paper on the mechan- isms of color vision by Lennie and D’Zmura (1988), the text on human color vision by Kaiser and Boynton (1996), the more general text on the founda- tions of vision by Wandell (1995), the comprehensive treatment by Palmer (1999), and edited collections on color vision by Backhaus et al. -

Phototransduction Mediated by Melanopsin in Intrinsically Photosensitive Retinal Ganglion Cells
C.A. Domínguez-Solís, J.A. Pérez-León: Phototransduction mediated by melanopsin Contents available at PubMed www.anmm.org.mx PERMANYER Gac Med Mex. 2015;151:709-20 www.permanyer.com GACETA MÉDICA DE MÉXICO REVIEW ARTICLE Phototransduction mediated by melanopsin in intrinsically photosensitive retinal ganglion cells Carlos Augusto Domínguez-Solís and Jorge Alberto Pérez-León* Department of Chemical-Biological Sciences, Institute of Biomedical Sciences, Universidad Autónoma de Ciudad Juárez, Chihuahua, Chih., México Abstract Melanopsin is the most recent photopigment described. As all the other opsins, it attaches in the retina as chromophore. Its amino acid sequence resembles more invertebrate opsins than those of vertebrates. The signal transduction pathway of opsins in vertebrates is based on the coupling to the G protein transducin, triggering a signaling cascade that results in the hyperpolarization of the plasma membrane. On the contrary, the photoreceptors of invertebrates activate the Gq protein pathway, which leads to depolarizing responses. Phototransduction mediated by melanopsin leads to the depolarization of those cells where it is expressed, the intrinsically photosensitive retinal ganglion cells; the cellular messengers and the ion channel type(s) responsible for the cells´ response is still unclear. Studies to elucidate the signaling cascade of melanopsin in heterologous expression systems, in retina and isolated/cultured intrinsically photosensitive retinal ganglion cells, have provided evidence for the involvement of protein Gq and phospholipase C together with the likely participation of an ion channel member of the transient receptor potential-canonical family, a transduction pathway similar to invertebrate photopigments, particularly Drosophila melanogaster. The intrinsically photosensitive retinal ganglion cells are the sole source of retinal inferences to the suprachiasmatic nucleus; thus, clarifying completely the melanopsin signaling pathway will impact the chronobiology field, including the clinical aspects. -
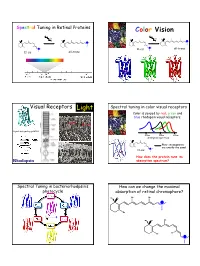
Color Vision Light
Spectral Tuning in Retinal Proteins Color Vision hν hν N N H H H H N all-trans N 11-cis 11-cis all-trans Visual Receptors Light Spectral tuning in color visual receptors Color is sensed by red, green and blue rhodopsin visual receptors. Rod Cone G-protein signaling pathway 400nm 500nm 600nm absorption spectrum Their chromophores H are exactly the same! 11-cis N How does the protein tune its Rhodopsin absorption spectrum? Spectral Tuning in bacteriorhodpsin’s How can we change the maximal photocycle absorption of retinal chromophore? Me Me Me Me N H Me Me Me Me N H Me Me Me Me Me Me Me H N 1 Excitation energy determines the Electronic Absorption maximal absorption Me Me Me Me π π* N - H S1 Me Absorption of light in the UV-VIS region of the S0 spectrum is due to Response excitation of electrons to higher energy levels. Me Me Me 13 7911 15 N H Me Me π-π* excitation in polyenes π-π* excitation in polyenes π∗ π∗ π∗ π∗ ∆E π∗ E photon E π π π π π Ground state (S0) Excited state (S1) ∆E (excitation energy, band gap) = hν = hc/λ blue-shift red-shift π-π* excitation in polyenes Tuning the length of the conjugated backbone β-carotene O O Vitamin A2 (retinal II) Vitamin A1 (retinal I) Longer wavelength Short wavelength 2 Retinal I Retinal II OPSIN SHIFT: how protein tunes the absorption maximum of its chromophore. Maximal absorption of protonated retinal Schiff base in: Water/methanol solution: 440 nm bR: 568 nm rod Rh: 500 nm red receptor: 560 nm green receptor: 530 nm blue receptor: 426 nm Salmon: different retinals in different stages of life Electrostatics and opsin shift Electrostatics and opsin shift S S positive charge 2 positive charge 2 S2 S2 Me Me Me Me Me Me S1 S1 S1 S1 + + + + N N H H Me O S0 Me O S0 Me O Me O C C Asp (Glu) Asp (Glu) S0 S0 no protein in protein no protein in protein counterion counterion • The counterion stabilizes the positive charge of the chromophore.