Isolation, Screening and Growth Optimization of Antagonistic Bacillus Subtilis MS21 from Thengapattanam Estuary Against Plant Fungal Pathogens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kanniyakumari District
STRICT DSTRICT GOVERNMENT OF TAMIL NADU DEPARTMENT OF GEOLOGY AND MINING DISTRICT SURVEY REPORT FOR ROUGHSTONE KANNIYAKUMARI DISTRICT (Prepared as per Gazette Notification S.O 3611(E) Dated 25.07.2018 of Ministry of Environment, Forest and Climate Change MoEF & CC) Contents S.No Chapter Page No. 1.0 Introduction 1 2.0 Overview of Mining Activity in the District; 4 3.0 General profile of the district 6 4.0 Geology of the district; 11 5.0 Drainage of irrigation pattern 17 6.0 Land utilisation pattern in the district; Forest, Agricultural, 18 Horticultural, Mining etc 7.0 Surface water and ground water scenario of the district 22 8.0 Rainfall of the district and climate condition 22 9.0 Details of the mining lease in the district 25-31 10.0 Details of Royalty / Revenue received in the last three years 32 11.0 Details of Production of Minor Mineral in last three Years 33 12.0 Mineral map of the district 34 13.0 List of letter of intent (LOI) holder in the district along with its 35 validity 14.0 Total mineral reserve available in the district. 36 15.0 Quality / Grade of mineral available in the district 39 16.0 Use of mineral 40 17.0 Demand and supply of the mineral in the last three years 40 18.0 Mining leases marked on the map of the district 41 19.0 Details of the area where there is a cluster of mining leases viz., 43 number of mining leases, location (latitude & longitude) 20.0 Details of eco-sensitive area 43 21.0 Impact on the environment due to mining activity 45 22.0 Remedial measure to mitigate the impact of mining on the 47 environment -

Basic Needs of 39 Coastal Fishing Communities in Kanniyakumari District, Tamil Nadu, India Bay of Bengal Programme Bobpimm/1
BASIC NEEDS OF 39 COASTAL FISHING COMMUNITIES IN KANNIYAKUMARI DISTRICT, TAMIL NADU, INDIA BAY OF BENGAL PROGRAMME BOBPIMM/1 Mimeo Series BASIC NEEDS OF 39 COASTAL FISHING COMMUNITIES IN KANNIYAKUMARI DISTRICT, TAMIL NADU, INDIA A SURVEY TO INVESTIGATE AND PRIORITISE PROBLEMS REGARDING SERVICES AND INFRASTRUCTURE by ReneJ.C.Verduijn Associate Professional Officer (Fishery Resource Economist) Bay ofBengalProgramme BAY OF BENGAL PROGRAMME, Chennai, India 2000 ii Preface This document describes a survey of the basic needs of 39 coastal fishing communities of Kanniyakumari district, Tamil Nadu, India, as perceived by the communities. The survey investigated and prioritized the communities’ needs for basic services such as water,education and health care. The survey was a co-operative effort ofthe Tamil Nadu Department ofFisheries, the Coastal Peace and DevelopmentCommittee of the Kottar Diocese, and the Bay ofBengal Programme (FAO/UN). The survey was carried out during the first half of 1998 by two local enumerators in each village selected by the Coastal Peace and Development Committee. All the enumerators were together imparted training for a day by the BOBP on the conduct of interviews, and given questionnaires. The enumerators conducted group interviews with fisherfolk of the 39 communities, both men and women, about the status of local services. This document details the findings of the survey and the comments by the respondents. It is hoped that these are founduseful by various governmentagencies and the church in improving the status of basic services and infrastructure in coastal areas of Kanniyakumari district. The survey, and this report of the survey, are part of the BOBP’s effort in co-operation with the TamilNadu Department of Fisheries to improve fisheries management in Kanniyakumari district. -

Characteristics of Nutrients in the Estuaries of Kanyakumari District-Tamilnadu-India
International Journal of Scientific & Engineering Research, Volume 6, Issue 11, November-2015 191 ISSN 2229-5518 CHARACTERISTICS OF NUTRIENTS IN THE ESTUARIES OF KANYAKUMARI DISTRICT-TAMILNADU-INDIA. Dr.kokila.p1 and Rathika.s2 1Head of the department,Vivekanandha college of arts and science, Kanyakumari 2Department of science and Humanities,Rohini college of engineering and technology Kanyakumari District-TAMILNAD, [email protected] ABSTRACT: Water samples were collected in four major estuaries of Kanyakumari District to analyse the distribution of dissolved nutrients and it is said to be polluted. Water samples were analysed for physico-chemical parameters including pH, turbidity, electrical conductivity,total hardness, dissolved oxygen , BOD and anions were analysed like Ca, Mg, Fe, free ammonia, NO3-, SO42-, PO42-, F- and Cl- during the different seasons of monsoon period from August - January (2011-2012).The concentrations of most of the investigated parameters in the water sample from Manakudy(Station1), Rajakamangalam(Station2), Kadiyapattanam (Station 3), Thengapattanam (Station 4) estauries in Kanyakumari district were exceeded the permissible limit of WHO, CPHEEO water quality guidelines. Keywords: Estauary, Nitrate, Dissolved Oxygen, WHO, CPHEEO. —————————— —————————— Introduction Rivers are the main inland water resources for domestic, industrial and irrigation purposes and often carry large municipal sewage, industrial waste water discharges and seasonal runoff from agricultural land to the coastal region. It is for this reason that the river water is mostly enriched in nutrients compared to other environments. The four major rivers that flow in Kanyakumari district are, Thambaraparani, Valliyar,IJSER Pazhayar and Paraliyar. Thambaraparani river confluences with Arabian sea near Thangaipattanam estuary about 56 km, west of Kanyakumari District. -

Historicity Research Journal
ISSN: 2393-8900 Impact Factor : 1.9152(UIF) VolUme - 4 | ISSUe - 9 | may - 2018 HIStorIcIty reSearcH JoUrNal ________________________________________________________________________________________ HISTORICAL ENQUIRY OF COLACHEL Dr. Praveen O. K. Assistant Professor, Department Of History, Sree Kerala Varma College, Thrissur, Kerala, India. ABSTRACT Travancore was the princely state existed in South India. It was called by different name in different period, it is popular being Venad, Vanchidesam and Tiruadidesam. Venad is originally known as Vanavanad which Merans the abode of the Dedvas. This was later simplified into Venad. Vanchi Desam means either the land of treasure or the land Baboons. In Malayalam the name of Travancore was interpreted by ‘Thiruvithamkure’ and in Sankrit ‘Srivardhanapuri’. KEY WORDS: Colachel, Battle of Colachel, Marthanda Varma, The Dutch, De Lannoy, Trade relations, The Dutch East India Company. INTRODUCTION Marthanda Varma inherited the throne of Travancore formerly known as Venad,from King Ramavarma, his uncle. During the period of Marthanda Varma the war held in Colachel is significance than the others. This war raises the war power of the king. In 1740, the Dutch Governor says to Marthanda Varma,that he was going to capture Travancore to his control. It internal idea is that Dutch are thinking to make colony in Travancore. In the beginning,and the war came to end of August 7,1741. The war was the important turning point of Marthanda Varma and Travancore State. NEED FOR THE PRESENT STUDY Colachal is sea port town from the ancient past, served as an important commercial centre for the Cheras, Pandyas and even the Dutch. It is surrounded by small villege lioke Kottilpadu, on the south, Puthutheru on the east, Velliyakulam on the North and Kalimar on the West. -

Assessment of Water Quality Along the Coast of Arabian Sea S.P.R
International Journal of Modern Science and Technology Vol. 1, Issue 2, May 2016. Page 69-79. http://www.ijmst.co/ ISSN: 2456-0235 Research Article Assessment of water quality along the coast of Arabian Sea S.P.R. Kalaikathir 1, S. Begila David 2* 1Department of Chemistry, Womens’ Christian College, Nagercoil – 629 001. India. 2PG and Research Centre in Chemistry, Scott Christian College (Autonomous), Nagercoil – 629 003. India. *Corresponding author’s e-mail: [email protected] Abstract The present work was focused on the spatial and temporal variations of the coastal water quality from six shores along southwest coastline in Arabian sea in Kanyakumari District,Tamilnadu, India. The surface temperature was found varied from 21.2°C to 28.1°C, pH was varied from 7.66 to 8.9, conductivity, TDS, DO, CO 2, Alkalinity and nitrate remains almost same in all places in a particular season. Turbidity was ranged between 2 NTU to 11 NTU and maximum in Kanyakumari in all seasons. The observed DO was above 5 mg/l. Minimum BOD was observed in the present study. Carbon dioxide was found varied from 8 ppm to 25 ppm. Total alkalinity values lie within the maximum permissible limit. Both gross alpha and gross beta count were maximum in Muttom coastal water. Nutrient nitrate varied from 2 g/l to 4 g/l. Phosphate was much higher in Muttom in summer season. Sulphate was higher in Kanyakumari and Thengapattanam coast which indicates anthropogenically affected water quality. Keywords : Coastal water; Alkalinity; Turbidity; Spatial and temporal variation. Besides the natural changes which bring Introduction about variation in physical and chemical Water is one of the most important and parameters of water, human interventions also abundant compounds of the ecosystem. -

Prominent Muslim Jamaaths in Kanyakumari District
International Journal of Research ISSN NO:2236-6124 Prominent Muslim Jamaaths in Kanyakumari District A. SHAHILA BANU Ph.D. Research Scholar (Reg. No.11842) Department of History, Women’s Christian College, Nagercoil – 629 001. Affiliated to Manonmaniam Sundaranar University, Abishekapatti, Tirunelveli – 627 012, Tamil Nadu, India. Abstract Islam in Kanyakumari District has been enriched by Fakirs and saintly savants of Islam. Due to their selfless service, Islam has thrived in this part of the country. The spread and growth of Islam is conspicuous by the presence of 52 Jamaaths in this district. These Jamaaths speak of their antiquity and they contain a lot of information regarding their life style and culture. In Kanyakumari District there are 52 Jamaaths. Among them six Muslim Jamaaths are considered very large and prominent. They are, Thengapattanam Muslim Jamaath, Colachel Muslim Jamaath, Thiruvithancode Muslim Jamaath, Thuckalay Muslim Jamaath, Elankadai Muslim Jamaath at Kottar, Malik Dinar faithulmal Muslim Jamaath, Kottar, Thittuvilai Muslim Jamaath. Some other Jamaaths are steadily growing in strength, stature and popularity. Keywords: Muslim Jamaath, Kanyakumari District, Life style, culture Introduction Jamaath means a Sangam, Federation or group based on some Islamic Principles. So it is said that a life without Jamaath is meaningless. If a Muslim keeps himself away from the Jamaath it means that he excommunicates himself from Islam. The Muslims form a Jamaath where they live and solve the problems that may arise out of marriage, divorce and other disputes. The solution to the problems would be based on the tenets of Holy Quran and the Hathees. Kanyakumari District Muslim Jamaath Federation was founded in 1982 at Thiruvithancode. -

Ahtl-European STRUGGLE by the MAPPILAS of MALABAR 1498-1921 AD
AHTl-EUROPEAn STRUGGLE BY THE MAPPILAS OF MALABAR 1498-1921 AD THESIS SUBMITTED FDR THE AWARD OF THE DEGREE DF Sactnr of pitilnsopliQ IN HISTORY BY Supervisor Co-supervisor PROF. TARIQ AHMAD DR. KUNHALI V. Centre of Advanced Study Professor Department of History Department of History Aligarh Muslim University University of Calicut Al.garh (INDIA) Kerala (INDIA) T6479 VEVICATEV TO MY FAMILY CONTENTS SUPERVISORS' CERTIFICATE ACKNOWLEDGEMENT LIST OF MAPS LIST OF APPENDICES ABBREVIATIONS Page No. INTRODUCTION 1-9 CHAPTER I ADVENT OF ISLAM IN KERALA 10-37 CHAPTER II ARAB TRADE BEFORE THE COMING OF THE PORTUGUESE 38-59 CHAPTER III ARRIVAL OF THE PORTUGUESE AND ITS IMPACT ON THE SOCIETY 60-103 CHAPTER IV THE STRUGGLE OF THE MAPPILAS AGAINST THE BRITISH RULE IN 19™ CENTURY 104-177 CHAPTER V THE KHILAFAT MOVEMENT 178-222 CONCLUSION 223-228 GLOSSARY 229-231 MAPS 232-238 BIBLIOGRAPHY 239-265 APPENDICES 266-304 CENTRE OF ADVANCED STUDY DEPARTMENT OF HISTORY ALIGARH MUSLIM UNIVERSITY ALIGARH - 202 002, INDIA CERTIFICATE This is to certify that the thesis "And - European Struggle by the Mappilas of Malabar 1498-1921 A.D." submitted for the award of the degree of Doctor of Philosophy of the Aligarh Muslim University, is a record of bonafide research carried out by Salahudheen O.P. under our supervision. No part of the thesis has been submitted for award of any degree before. Supervisor Co-Supervisor Prof. Tariq Ahmad Dr. Kunhali.V. Centre of Advanced Study Prof. Department of History Department of History University of Calicut A.M.U. Aligarh Kerala ACKNOWLEDGEMENT My earnest gratitude is due to many scholars teachers and friends for assisting me in this work. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2014 [Price : Rs. 146.40 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 10-Q] CHENNAI, WEDNESDAY, MARCH 12, 2014 Maasi 28, Thiruvalluvar Aandu–2045 Part VI–Section 1 (Supplement) NOTIFICATIONS BY HEADS OF DEPARTMENTS, ETC. TAMIL NADU NURSES AND MIDWIVES COUNCIL, CHENNAI. (Constituted under Tamil Nadu Act III of 1926) TAMIL NADU MIDWIVES COUNCIL ELECTORAL ROLL THE YEAR 1998 [ 1 ] DTP—VI-1 Sup. (10-Q)—1 2 REGISTERED MIDWIVES FOR THE YEAR 1998 Regn.No: Name of the Particulars Date of Address Candidate Regn. 46718 E.Daisy Govt.of Tamilnadu 20th 99,Murugan Cert.No:3602 trained at Jan.1998. Illam,Chennimalai 638 Deepavi Institute of 051,Erode District Medical Technology Training Centre,Erode -9 from 1st December 1993 to 30th November 1996.Exam December 1996. 46719 Antony Mary Govt. of Tamilnadu 20th D/o.S.Dharmaraj Dharmaraj cert.No:3506 trained at Jan.1998. Aranganer Villai Alencode Rajapalayam Nurses Post,Kanyakumari Dist.629 Training 802. Institute,Rajapalayam from 1st July 1993 to 30th june 1996.Exam May 1996. 46720 Aleyamma K.A. Govt of Andhara Pradesh 20th Kurisinkal House,CMC- Cert.trained at Vijaya Jan.1998. 23,Chirthala Post,Alapuzha School of District,Kerala. Nursing,Guntur from January 1994 to January 1997.Exam January 1997.Primary Registration with Andhra Pradesh Nursing Council under No:23220 dated 26th June 1997. 46721 P.Jeeva Govt of Andhara Pradesh 20th Veerangkuppam Cert.trained at Jan.1998. Village,Kumaramargh Priyadarshini School of Post,Thuthipeti Via 635 Nursing,Rajahmundry 811. -

Kanyakumari District
KANYAKUMARI DISTRICT 1 KANYAKUMARI DISTRICT 1. Introduction i) Geographical location of the district Kanyakumari is the Southern most West it is bound by Kerala. With an area of district of Tamil Nadu. The district lies 1672 sq.km it occupies 1.29% of the total between 77 o 15 ' and 77 o 36 ' of the Eastern area of Tamil Nadu. It ranks first in literacy Longitudes and 8 o 03 ' and 8 o 35 ' of the among the districts in Tamil Nadu. Northern Latitudes. The district is bound by Tirunelveli district on the North and the East. ii) Administrative profile The South eastern boundary is the Gulf of The administrative profile of Mannar. On the South and the South West, Kanyakumari district is given in the table the boundaries are the Indian Ocean and the below Arabian sea. On the west and North Name of the No. of revenue Sl. No. Name of taluk No. of firka division villages 1 Agastheeswaram 4 43 1 Nagercoil 2 Thovalai 3 24 3 Kalkulam 6 66 2 Padmanabhapuram 4 Vilavancode 5 55 Total 18 188 ii) 2 Meteorological information and alluvial soils are found at Based on the agro-climatic and Agastheeswaram and Thovalai blocks. topographic conditions, the district can be divided into three regions, namely: the ii) Agriculture and horticulture uplands, the middle and the low lands, which are suitable for growing a number of crops. Based on the agro-climatic and The proximity of equator, its topography and topographic conditions, the district can be other climate factors favour the growth of divided into three regions, namely:- various crops. -

Kanyakumari District Statistical Handbook – 2016
Kanyakumari District Statistical Handbook – 2016 Preface Salient Features District Profile 1. Area &Population 2. Climate & Rainfall 3. Agriculture 4. Irrigation 5. Animal Husbandary 6. Banking & Insurance 7. Co-Operative Societies 8. Civil Supplies 9. Communications 10. Electricity 11. Education 12. Fisheries 13. Handloom 14. Handicrafts 15. Health & Family Welfare 16. Housing 17. Industries 18. Factories 19. Local Bodies 20. Labour & Employment 21. Legal services 22. Libraries 23. Mining & Quarrying 24. Manufacturing 27. Non-Conventional 25. Medical Services 26. Motor Vehicles Energy 28. Police & Prison 29. Public Health 30. Printing & Publications 31. Prices Indices 32. Quality Control 33. Registration 36. Recreation & Cultural 34. Repair & Services 35. Restaurants & Hotels Services 39. Scientific Research 37. Social Welfare 38. Sanitary Services Services 40. Storage Facilities 41. Textiles 42. Trade & Commerce 43. Transport 44. Tourism 45. Vital Statistics 46. Voluntary Services 47. Waterworks & Supply 48. Rubber Study DEPUTY DIRECTOR OF STATISTICS KANNIYAKUMARI DISTRICT PREFACE The District Statistical Hand Book is prepared and published by our Department every year. This book provides useful data across various departments in Kanniyakumari District. It contains imperative and essential statistical data on different Socio-Economic aspects of the District in terms of statistical tables and graphical representations. This will be useful in getting a picture of Kanniyakumari’s current state and analyzing what improvements can be brought further. I would liketo thank the respectable District Collector Sh. SAJJANSINGH R CHAVAN, IAS for his cooperation in achieving the task of preparing the District Hand Book for the year 2015-16 and I humbly acknowledge his support with profound gratitude. The co-operation extended by the officers of this district, by supplying the information presented in this book is gratefully acknowledged. -
Kanyakumari Sl
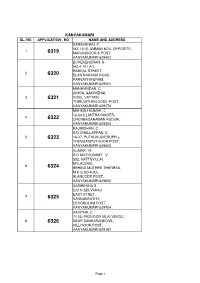
KANYAKUMARI SL. NO. APPLICATION . NO NAME AND ADDRESS SARAVANAN. P NO.11/12, AMMAN KOIL OPPOSITE, 1 6319 MARUNGOOR & POST, KANYAKUMARI 629402 BUVENENDRAN. R NO.4-101 A/1, RABEAL STREET, 2 6320 ELANTHAIYADI ROAD, PARVATHIPURAM, KANYAKUMARI 629001 MANIKANDAN. C 23/97A, AAKIVIZHAI, 3 6321 KOVIL VATTAM, THIRUVITHAN CODE POST, KANYAKUMARI 629174 MAHESH KUMAR. C 12-24,ILLANTHAI NAGER, 4 6322 CHENBAGARAMAN PUDUR, KANYAKUMARI 629304 RAJIMOHAN. C S/O CHELLAPPAN. S 5 6323 16-47, PUTHUKUDIERUPPU, THENGAMPUTHOOR POST, KANYAKUMARI 629603 KUMAR . M S/O MUTHUSAMY . V 332, KATTUVILLAI, MYLACODE, 6 6324 BEHIND MOTHER THERESA, M.H.S SCHOOL, ALANCODE POST, KANYAKUMARI 629802 SASIREKHA.S D/O N.SELVARAJ EAST STRET, 7 6325 KANNANPATHY, DEVOKULAM POST, KANYAKUMARI 629704 KAVITHA. C 7/103, PAZHOOR VILAI VEEDU, 8 6326 NEAR SANKARANKOVIL, KILLIYOOR POST, KANYAKUMARI 629187 Page 1 RAJKUMAR. V S/O S.VELAYUTHAM, 9 6327 SOUTH KANNANKULAM, PARAKKAI POST, KANYAKUMARI 629601 PRINCE R S/O A.KAPUR 36-4/44 SALAIPUTHOOR, 10 6328 MYLAUDY WAYKULASEKAPAPURAM POST, KANYAKUMARI 629403 SUJITHA LEGHA.K.P D/O KOLAPPAN.P KARTHICK BHAVAN, 11 6329 VANIAN VILLAI, MELANGALAM, PUTHUKKADAI POST, KANYAKUMARI 629171 MONIKAN DAN. R S/O. RAJAMONI. M 12 6330 PALATTIN KAVAI, AMMANDIVILAI POST, KANYAKUMARI 629204 SUDALAI MONI. M S/O P.MARY, DOOR NO.5/24, 13 6331 SIVASUBRAMANIAPURAM, JAMESTOWN POST, KANYAKUMARI 629401 PRABAKAR . C S/O CHANDRAN . P RO-93, NEAR PILLAYAR KOIL, 14 6332 KURATHIYARAI, AZHAGIAPANDIAPURAM POST, KANYAKUMARI 629851 AHILA. T.M D/O GNANAM. M DOOR NO; NEW NO 9/73, 15 6333 ERANIEL KONAM, KEEZHAMAIANKUCHI, NEYYOOR POST, KANYAKUMARI 629802 ANAND. V 162B ARATTU ROAD, S.M.R.V. -

National Assessment of Shoreline Changes Along Indian Coast
National Assessment of Shoreline changes along Indian Coast R. S. Kankara, M. V. Ramana Murthy & M. Rajeevan Status report for years 1990-2016 Ministry of Earth Sciences National Centre for Coastal Research Chennai-600100 July- 2018 National Assessment of Shoreline changes along Indian Coast: Status report for 26 years (1990 - 2016) NCCR PROJECT TEAM MEMBERS 1 Dr. M. V Ramana Murthy, Director & Scientist-G, NCCR 2 Dr. R. S Kankara, Group Head & Scientist F, CP & SM Group, NCCR 3 Mr. S Chenthamil Selvan, Project Scientist-I 4 Mr. K Prabhu, Project Scientist-I 5 Mr. Rajan B, Senior Research Fellow 6 Mr. Arockiraj, Senior Research Fellow 7 Mrs. S. Dhanalakshmi, Senior Research Fellow 8 Miss. Padmini, Senior Research Fellow 9 Dr. V. Noujas, Project Scientist-I 10 Miss. M Uma Maheswari, Senior Research Fellow 11 Mr. Sathish Sathasivam, Project Scientist-I EXPERT COMMITTEE 1 Dr. Shailesh Nayak, Former Secretary, MoES New Delhi Chairman 2 Dr. Ashok Kumar Saxena, Project Director, ICZMP, Gujarat Member 3 Dr. Ajit Pattnaik, Project Director (Retd), ICZMP, Odisha Member 4 Sh. Arvind Kumar Nautiyal, Director, MoEF & CC, New Delhi Member 5 Sh. Sanjay Kumar Singh, Director, CWC, MoWR, RD&GR, New Delhi Member DISCLAIMER This report is part of series of reports that includes text summarizing methods, results, in addition to maps illustrating zones of shoreline change. Zones of shoreline change are being published for the purpose of coastline characterization. The report / maps are not intended to be equated to either as revenue maps of the respective State/ UT/ Government agencies or as the topographic maps of the Survey of India.