Vibrio Cholerae Virulence Regulator-Coordinated Evasion of Host Immunity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Turning on Virulence: Mechanisms That Underpin the Morphologic Transition and Pathogenicity of Blastomyces
Virulence ISSN: 2150-5594 (Print) 2150-5608 (Online) Journal homepage: http://www.tandfonline.com/loi/kvir20 Turning on Virulence: Mechanisms that underpin the Morphologic Transition and Pathogenicity of Blastomyces Joseph A. McBride, Gregory M. Gauthier & Bruce S. Klein To cite this article: Joseph A. McBride, Gregory M. Gauthier & Bruce S. Klein (2018): Turning on Virulence: Mechanisms that underpin the Morphologic Transition and Pathogenicity of Blastomyces, Virulence, DOI: 10.1080/21505594.2018.1449506 To link to this article: https://doi.org/10.1080/21505594.2018.1449506 © 2018 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group© Joseph A. McBride, Gregory M. Gauthier and Bruce S. Klein Accepted author version posted online: 13 Mar 2018. Submit your article to this journal Article views: 15 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=kvir20 Publisher: Taylor & Francis Journal: Virulence DOI: https://doi.org/10.1080/21505594.2018.1449506 Turning on Virulence: Mechanisms that underpin the Morphologic Transition and Pathogenicity of Blastomyces Joseph A. McBride, MDa,b,d, Gregory M. Gauthier, MDa,d, and Bruce S. Klein, MDa,b,c a Division of Infectious Disease, Department of Medicine, University of Wisconsin School of Medicine and Public Health, 600 Highland Avenue, Madison, WI 53792, USA; b Division of Infectious Disease, Department of Pediatrics, University of Wisconsin School of Medicine and Public Health, 1675 Highland Avenue, Madison, WI 53792, USA; c Department of Medical Microbiology and Immunology, University of Wisconsin School of Medicine and Public Health, 1550 Linden Drive, Madison, WI 53706, USA. -

Evaluation of Virulence Factors and Antibiotic Resistance Patterns in Clinical Urine Isolates of Klebsiella Pneumoniae in Semnan, Iran
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by eprints Iran University of Medical Sciences Jundishapur J Microbiol. 2018 July; 11(7):e63637. doi: 10.5812/jjm.63637. Published online 2018 June 9. Research Article Evaluation of Virulence Factors and Antibiotic Resistance Patterns in Clinical Urine Isolates of Klebsiella pneumoniae in Semnan, Iran Ali Jazayeri Moghadas,1 Farzaneh Kalantari,2 Mohammad Sarfi,3 Soroush Shahhoseini,3 and Shiva Mirkalantari4, * 1Bacteriology and Virology Department, Medicine Faculty, Semnan University of Medical Sciences, Semnan, Iran 2Laboratory Technical Officer, Shafa Hospital, Semnan, Iran 3Student Research Committee, Semnan University of Medical Sciences, Semnan, Iran 4Department of Microbiology, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran *Corresponding author: Shiva Mirkalantari, Department of Microbiology, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran. E-mail: [email protected] Received 2017 November 04; Revised 2018 April 19; Accepted 2018 April 29. Abstract Background: Klebsiella pneumoniae as an opportunistic pathogen can be the cause of a range of nosocomial and community - ac- quired infections. Many virulence factors help these bacteria overcome an immune system and cause various diseases. K1 and K2 capsular antigens, also magA, wcaG, and rmpA are well - known K. pneumoniae virulence factors. Klebsiella pneumoniae has been re- vealed to have the ability to acquire resistance to many antibiotics, which cause treatment failure. Objectives: This study aimed at determining the prevalence of magA, wcaG, rmpA, Capsular type K1, Capsular type K2, TEM, and SHV in K. pneumoniae isolates. Methods: A total of 173 non - duplicate K. -

Chlamydia Trachomatis Strains and Virulence: Rethinking Links to Infection Prevalence and Disease Severity
SUPPLEMENT ARTICLE Chlamydia trachomatis Strains and Virulence: Rethinking Links to Infection Prevalence and Disease Severity Gerald I. Byrne Department of Molecular Sciences, University of Tennessee Health Science Center, Memphis An unanswered question concerning prevalence and disease severity of Chlamydia trachomatis genital infection is whether more prevalent strains or strains more likely to cause serious disease complications are causally associated with specific virulence attributes. The major method for distinguishing chlamydial strains is based on differences in the major outer membrane protein (MOMP). A subset of MOMP serovars (D and E serovars) are easily the most prevalent strains identified worldwide, but MOMP serovar and genovar analyses have not yielded consistent strain-dependent virulence distinctions. Expansion of the definitions of chlamydial strains beyond the MOMP paradigm are needed to better understand virulence properties for this pathogen and how these properties reflect disease severity. Substantive genetic and phenotypic differences have emerged for the 2 major C. trachomatis pathobiotypes associated with either trachoma or sexually transmitted diseases, but differences within the sexually transmitted disease group have not yielded reliable disease severity attributes. A number of candidate virulence factors have been identified, including the polymorphic outer membrane autotransporter family of proteins, the putative large cytotoxin, type III secretion effectors, stress response proteins, and proteins or other regulatory factors produced by the cryptic plasmid. Continued work on development of a chlamydial gene transfer system and application of genomic approaches to large collections of clinical isolates will be required to associate key chlamydial virulence factors with prevalence and disease severity in a definitive way. Identification and sorting of different Chlamydia tra- ease in women or other serious complications of chla- chomatis genital tract isolates have been central to ep- mydial genital tract infection [1]. -

Virulence Factors and Their Mechanisms of Action: the View from a Damage–Response Framework Arturo Casadevall and Liise-Anne Pirofski
S2 Q IWA Publishing 2009 Journal of Water and Health | 07.S1 | 2009 Virulence factors and their mechanisms of action: the view from a damage–response framework Arturo Casadevall and Liise-anne Pirofski ABSTRACT The virulence factor concept has been a powerful engine in driving research and the intellectual Arturo Casadevall (corresponding author) Liise-anne Pirofski flow in the fields of microbial pathogenesis and infectious diseases. This review analyzes Division of Infectious Diseases, virulence factors from the viewpoint of the damage–response framework of microbial Department of Medicine and the Department of Microbiology and Immunology of the Albert pathogenesis, which defines virulence factor as microbial components that can damage a Einstein College of Medicine, 1300 Morris Park Avenue, susceptible host. At a practical level, the finding that effective immune responses often target Bronx NY 10461, USA virulence factors provides a roadmap for future vaccine design. However, there are significant Tel.: +1 718 430 2215 limitations to this concept, which are rooted in the inability to define virulence and virulence Fax: +1 718 430 8968 E-mail: [email protected] factors in the absence of host factors and the host response. In fact, this concept appears to work best for certain types of bacterial pathogens, being less well suited for viruses and commensal organisms with pathogenic potential. Key words | damage–response framework, microbe, pathogen, pathogenicity, virulence, virulence factor INTRODUCTION The idea that pathogenic microbes are endowed with understanding of microbial virulence. In this paper, we certain components that confer upon them the capacity review the historical context for the emergence of the for virulence is the central theme of the virulence factor virulence factor concept and then consider it from concept. -

Relationship of Virulence Factors and Clinical Features in Keratitis Caused by Pseudomonas Aeruginosa
Immunology and Microbiology Relationship of Virulence Factors and Clinical Features in Keratitis Caused by Pseudomonas aeruginosa Naoko Oka,1 Takashi Suzuki,1 Eri Ishikawa,1 Satoshi Yamaguchi,1,2 Naoki Hayashi,3 Naomasa Gotoh,3 and Yuichi Ohashi1 1Department of Ophthalmology, Ehime University Graduate School of Medicine, Toon, Ehime, Japan 2ROHTO Pharmaceutical Co., Ltd., Osaka, Japan 3Department of Microbiology and Infection Control Science, Kyoto Pharmaceutical University, Yamashina, Kyoto, Japan Correspondence: Takashi Suzuki, PURPOSE. To examine bacterial virulence factors in Pseudomonas aeruginosa isolates from Department of Ophthalmology, contact lens (CL) wearers and non–CL wearers with P. aeruginosa keratitis, and to investigate Ehime University Graduate School of relationships between virulence factors and clinical features of keratitis. Medicine, Shitsukawa, Toon, Ehime 791-0295, Japan; METHODS. The study involved 25 subjects including 18 CL and 7 non–CL-related P. aeruginosa [email protected] keratitis patients. Slit-lamp photographs of all subjects were captured, and the focus Submitted: June 24, 2015 occupancy ratio (FOR) was defined as the total focus area/entire cornea area, using image Accepted: September 22, 2015 processing software. Twenty-five clinical P. aeruginosa isolates from keratitis were assessed for protease production, elastase production, biofilm formation, bacterial swimming and Citation: Oka N, Suzuki T, Ishikawa E, swarming motility, cell surface hydrophobicity, and genes encoding the type III secretion et al. Relationship of virulence factors and clinical features in keratitis caused system (TTSS) effectors (ExoU and ExoS). by Pseudomonas aeruginosa. Invest RESULTS. Ring abscess was found in 9 of 18 CL-related P. aeruginosa keratitis cases (CL[þ] Ophthalmol Vis Sci. -

Bacterial Determinants of Importance in the Virulence of Gallibacterium Anatis in Poultry Gry Persson and Anders M Bojesen*
Persson and Bojesen Veterinary Research (2015) 46:57 DOI 10.1186/s13567-015-0206-z VETERINARY RESEARCH REVIEW Open Access Bacterial determinants of importance in the virulence of Gallibacterium anatis in poultry Gry Persson and Anders M Bojesen* Abstract Gallibacterium anatis, a member of the Pasteurellaceae family, constitute a part of the normal micro-flora of the upper respiratory tract and the lower genital tract in chickens. However, increasing evidence indicate that G. anatis is also associated with a wide range of pathological changes, particularly in the reproductive organs, which leads to decreased egg production, lowered animal welfare and increased mortality. As a recently defined opportunistic pathogen limited focus has been placed on the pathogenesis and putative virulence factors permitting G. anatis to cause disease. One of the most studied virulence determinants is a large RTX-like toxin (GtxA), which has been demonstrated to induce a strong leukotoxic effect on avian macrophages. A number of fimbria of different sizes and shapes has been described. Particularly fimbriae belonging to the F17-like family appears to be common in a diverse selection of G. anatis strains. Mutants lacking the FlfA fimbria were severely attenuated in experimentally infected chickens. Additional characteristics including the ability to express capsular material possibly involved in serum resistance; secretion of metalloproteases capable of degrading immunoglobulins, and hemagglutinins, which may promote biofilm formation are all factors likely linked to the virulence of G. anatis. A major advantage for the study of how G. anatis interact with its host is the ability to perform biologically relevant experimental infections where natural routes of exposure allows reproduction of lesions observed during spontaneous infections. -
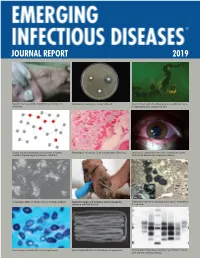
2019 EID Journal Report
JOURNAL REPORT 2019 Swollen eye caused by morbillivirus infection in Fosfomycin resistance in Escherichia coli. Severe fever with thrombocytopenia syndrome virus wild boar. in Haemaphysalis longicornis tick. Sexual network showing transmission of highly Pathologic findings for fetus infected with Zika virus. Left eye of a physician from the United States who resistant Neisseria gonorrhoeae infections. contracted Ebola virus disease in Liberia. Cytopathic effect of Nipah virus in infected patient. Nasal discharge in dromedary camel caused by Differing virulence of Candida auris and C. haemulonii infection with MERS-CoV. in a mouse. Yeast in patient with African histoplasmosis. Adult Diphyllobothrium nihonkaiense tapeworm. Immunoblot of protease-resistant prion from moose with chronic wasting disease. Message from the Editor-in-Chief CDC’s Emerging Infectious Diseases (EID)—a high-impact, open-access, peer-reviewed journal available at no cost to readers online and in print—publishes articles that discuss disease emer- gence, prevention, and elimination. With its December 2018 issue, EID passed a landmark— publication of its 10,000th article since its launch in 1995. This 2019 Emerging Infectious Dis- eases Journal Report highlights key metrics and activities that show EID is successfully reaching its core public health audiences. Several metrics reveal how valuable the journal’s content is for readers. First, EID’s online page views exceeded 8,413,483 in 2018. Second, EID’s most recent journal impact factor score* of 7.42, based on how often its articles are cited in other scientific literature, ranked first among open-access journals and fourth among all 88 infectious disease journals tracked. Third, EID’s content, available from PubMed Central, the United States National Library of Medicine’s digital repository, was ac- cessed 3,427,212 times—up 420,626, from 2018 and a ninefold increase since 2009, the first year those data were available. -

NIH Public Access Author Manuscript Nat Rev Microbiol
NIH Public Access Author Manuscript Nat Rev Microbiol. Author manuscript; available in PMC 2014 October 22. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Nat Rev Microbiol. 2014 April ; 12(4): 274–288. doi:10.1038/nrmicro3235. Bordetella pertussis pathogenesis: current and future challenges Jeffrey A. Melvin1, Erich V. Scheller1, Jeff F. Miller2, and Peggy A. Cotter1,* 1Department of Microbiology and Immunology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA 2Department of Microbiology, Immunology & Molecular Genetics, University of California, Los Angeles, Los Angeles, CA, USA Abstract Pertussis, or whooping cough, has recently reemerged as a major public health threat despite high levels of vaccination against the etiological agent, Bordetella pertussis. In this Review, we describe the pathogenesis of this disease, with a focus on recent mechanistic insights into virulence factor function. We also discuss the changing epidemiology of pertussis and the challenges of vaccine development. Despite decades of research, many aspects of B. pertussis physiology and pathogenesis remain poorly understood. We highlight knowledge gaps that must be addressed to develop improved vaccines and therapeutic strategies. Pertussis is a highly contagious respiratory disease that is transmitted directly from human to human1, most likely via aerosolized respiratory droplets. The primary causative agent, Bordetella pertussis, is a Gram-negative bacterium that was first described by Bordet and 2 Gengou in 1906 . The closely related bacterium Bordetella parapertussisHu is responsible for a minority of cases (approximately 14%) and is less capable of causing severe disease3. Both B. pertussis and B. parapertussisHu are human-specific, and phylogenetic analyses indicate that they evolved from Bordetella bronchiseptica or a B. -

Taylor & Francis and EIFL Open Access Agreement
Taylor & Francis and EIFL Open Access agreement: participating journals Please find below a list of all Taylor & Francis journals offering discounted or waived article publishing charges (APCs) to EIFL network countries. Find out more about how you can publish open access using the EIFL APC agreement on our Author Services website. Please note that APCs are not payable for articles published in Diamond journals, which are highlighted bold in this list. Food and Agricultural Immunology Biomaterial Investigations in Dentistry Artificial Cells, Nanomedicine, and Biotechnology Annals of Medicine Acta Oto-Laryngologica Case Reports Case Reports in Plastic Surgery and Hand Surgery Computer Assisted Surgery Drug Delivery Journal of Enzyme Inhibition and Medicinal Chemistry International Journal of Hyperthermia Journal of Immunotoxicology Journal of Drug Assessment Journal of Medical Economics Pharmaceutical Biology Scandinavian Journal of Primary Health Care Renal Failure Adipocyte Bioengineered Cell Adhesion & Migration Channels Communicative & Integrative Biology GM Crops & Food Gut Microbes Health Systems & Reform mAbs Nucleus OncoImmunology Prion Virulence International Journal of Food Properties Journal of Integrative Environmental Sciences Mathematical and Computer Modelling of Dynamical Systems Cogent Arts & Humanities Cogent Business & Management Cogent Education Cogent Economics & Finance Cogent Engineering Sustainable Environment Cogent Food & Agriculture RMS: Research in Mathematics & Statistics Cogent Medicine Cogent Psychology Cogent -

Taylor & Francis Standard Reference Style |
Taylor & Francis Standard Reference Style | NLM National Library of Medicine (NLM) is a member of the National Information Standards Organization and its various published standards have been adopted for the Library’s MEDLINE/PubMed database. See https://www.nlm.nih.gov/citingmedicine/ for complete guidance, including less frequently used reference types. Please note that Taylor & Francis does not require the inclusion of all optional elements, so you should match the samples below. If you are using this reference style the EndNote output style can be found at http://endnote.com/downloads/style/tf-standard-nlm Version 2.0 Date of issue: 14 May 2013 Date of version: 10 January 2018 Update in this version: dataset model added Contents In the text .......................................................................................................................................... 2 Tables and figures ............................................................................................................................ 3 Reference list .................................................................................................................................... 3 Journal .......................................................................................................................................... 3 Book .............................................................................................................................................. 5 Conference ................................................................................................................................... -

Chapter 1. Bacteria: Pathogenicity Factors
I.1. BACTERIA: PATHOGENICITY FACTORS – 27 Chapter 1. Bacteria: Pathogenicity factors This chapter provides guidance on topics and issues relevant to the risk/safety assessment of commercial environmental applications involving genetically engineered micro-organisms, especially bacteria. It explores the important aspects in bacteria for causing adverse human health effects, and how this knowledge can be used in biosafety regulatory assessment. It contains information on bacterial pathogenicity (general considerations, factors and determinants, genetics and molecular biology), and also elements on assessing potential for bacteria-mediated adverse human health effects. The chapter was prepared by the OECD Working Group on the Harmonisation of Regulatory Oversight in Biotechnology, Sub-working Group on Micro-organisms, with the Netherlands and Canada having served as lead countries. It was initially issued in September 2011. SAFETY ASSESSMENT OF TRANSGENIC ORGANISMS: OECD CONSENSUS DOCUMENTS, VOLUME 5 © OECD 2016 28 – I.1. BACTERIA: PATHOGENICITY FACTORS General considerations for bacterial pathogenicity This chapter provides guidance on the concept of bacterial pathogenicity in the context of risk/safety assessment of deliberate release of “genetically engineered”, or “genetically modified”,1 micro-organisms intended for commercial environmental applications (e.g. bioremediation, biosensors, biofertilisers, biopesticides, biomining, biomass conversion or oil recovery). It is limited in scope to bacteria that may exhibit properties pathogenic to human beings. Not included in the scope are environmental releases of known (potential) pathogens, e.g. vaccine strains. The chapter explores the factors that are important in bacteria for causing adverse human health effects and assesses how this knowledge can be used in risk/safety assessment of environmental applications of bacteria. -

The Coxiella Burnetii Qph1 Plasmid Is a Virulence Factor for Colonizing
bioRxiv preprint doi: https://doi.org/10.1101/2020.03.22.001875; this version posted March 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 The Coxiella burnetii QpH1 plasmid is a virulence factor for 2 colonizing bone marrow-derived murine macrophages 3 4 Shengdong Luo1,2¶, Zhihui Sun2¶, Huahao Fan1¶, Shanshan Lu1,3&, Yan Hu3&, 5 Ruisheng Li3&, Xiaoping An1&, Yigang Tong1*, Lihua Song1,2* 6 7 1 Beijing Advanced Innovation Center for Soft Matter Science and Engineering, 8 College of Life Science and Technology, Beijing University of Chemical Technology, 9 Beijing, China 10 11 2 State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology 12 and Epidemiology, Beijing, China 13 14 3 Research Center for Clinical Medicine, The Fifth Medical Center of PLA General 15 Hospital, Beijing, China 16 17 * Corresponding author 18 Email: [email protected] (LS); [email protected] (YT). 19 20 ¶ These authors contributed equally to this work. 21 & These authors also contributed equally to this work. 22 bioRxiv preprint doi: https://doi.org/10.1101/2020.03.22.001875; this version posted March 25, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license.