Appendix A-III Substances Which Need Not Be Reported Unless Manufactured by the Facility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
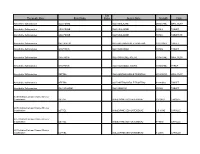
Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

Prevention and Management of Intravesical BCG-Related Lower Urinary Tract Symptoms with Prophylactic Pentosan Polysulphate In
Prevention and Management of Intravesical BCG-related Lower Urinary Tract Symptoms with Prophylactic Pentosan Polysulphate in Patients with Non-Muscle-Invasive Bladder Cancer: A Randomized Controlled Trial Samer Shamout, Simon Tanguay, Maurice Anidjar, Lysanne Campeau CQI Research Grant 2017 INTRODUCTION METHODS / INTERVENTIONS PATIENT IMPACT Study Design • Bacillus Calmette-Guerin (BCG) remains the most effective • Phase 2, double-blind, randomized, placebo-controlled, parallel-group • Improve patient experience by identifying predictor markers prophylactic treatment of intermediate and high-risk non- study rendering patients at higher risk of developing BCG-related muscle invasive bladder cancer. • Multicentre trial involving two partner sites: (Jewish General Hospital, LUTS. McGill University Health Centre) • 30 to 60% of bladder cancer patients experienced systemic • This will guide patient counselling before treatment to inform Outcomes them about associated risks and benefits of the BCG and local adverse events of variable severity following Primary outcomes (change from baseline to end of treatment): treatment. intravesical BCG therapy • Mean change in number of urgency episodes/24hr (3-day bladder diary) • Only 16 to 29% of patients completed all the three-year BCG • Mean change in ICIQ-LUTSqol score • We will determine the role of prophylactic PPS to prevent these treatment regimen. • Mean change in OAB-V8 score LUTS from occurring, particularly if patients are considered at Secondary outcomes (change from baseline to end of treatment): higher risk, and therefore improve patient experience. • Guidelines recommend medications, such as oxybutynin • Mean change in VAS score only phenazopyridine, and propantheline bromide for short term • Mean change in urinary inflammatory markers (TRAIL, IFN, IL-2, IL-10) • The improvement measurements are validated in the symptomatic relief. -

Postmenopausal Pharmacotherapy Newsletter
POSTMENOPAUSAL PHARMACOTHERAPY September, 1999 As Canada's baby boomers age, more and more women will face the option of Hormone Replacement Therapy (HRT). The HIGHLIGHTS decision can be a difficult one given the conflicting pros and cons. M This RxFiles examines the role and use of HRT, as well as newer Long term HRT carries several major benefits but also risks SERMS and bisphosphonates in post-menopausal (PM) patients. which should be evaluated on an individual and ongoing basis MContinuous ERT is appropriate for women without a uterus HRT MWomen with a uterus should receive progestagen (at least 12 HRT is indicated for the treatment of PM symptoms such as days per month or continuous low-dose) as part of their HRT vasomotor disturbances and urogenital atrophy, and is considered MLow-dose ERT (CEE 0.3mg) + Ca++ appears to prevent PMO primary therapy for prevention and treatment of postmenopausal MBisphosphinates (e.g. alendronate, etidronate) and raloxifene are osteoporosis (PMO).1 Contraindications are reviewed in Table 2. alternatives to HRT in treating and preventing PMO Although HRT is contraindicated in women with active breast or M"Natural" HRT regimens can be compounded but data is lacking uterine cancer, note that a prior or positive family history of these does not necessarily preclude women from receiving HRT.1 Comparative Safety: Because of differences between products, some side effects may be alleviated by switching from one product Estrogen Replacement Therapy (ERT) 2 to another, particularly from equine to plant sources or from oral to Naturally secreted estrogens include: topical (see Table 3 - Side Effects & Their Management). -

Impact of Tamsulosin, Tolterodine and Drug-Combination On
African Journal of Urology (2017) 23, 28–32 African Journal of Urology Official journal of the Pan African Urological Surgeon’s Association web page of the journal www.ees.elsevier.com/afju www.sciencedirect.com Original article Impact of Tamsulosin, Tolterodine and drug-combination on the outcomes of lower urinary tract symptoms secondary to post-ureteroscopy ureteral stent: A prospective randomized controlled clinical study a b,∗ a a O. Abdelkader , K. Mohyelden , M.H. Sherif , A.H. Metwaly , b c d a H. Aldaqadossi , A. Shelbaya , H. Khairy , A. Elnashar a Department of Urology, Suez Canal University, Ismailia, Egypt b Department of Urology, Fayoum University, Fayoum, Egypt c Student Hospital, Cairo University, Cairo, Egypt d Department of Urology, Cairo University, Cairo, Egypt Received 17 March 2016; received in revised form 23 June 2016; accepted 23 June 2016 Available online 18 November 2016 KEYWORDS Abstract Anticholinergics; Objectives: To compare the role of alpha-blocker (Tamsulosin) monotherapy, anticholinergic (Tolterodine) ␣-Adrenergic blockers; monotherapy or combination of both drugs versus analgesics in improving post-ureteroscopy (URS) lower Ureter stent; urinary tract symptoms related to double-J ureteral stent. Ureteroscopy Patients and methods: Between January 2009 and June 2013, 160 consecutive patients with ureteric stones were included in this study at 2 tertiary care centers’. Patients were randomized into 4 groups; group A (n = 40) received 0.4 mg Tamsulosin once a day, group B (n = 40) received 4 mg Tolterodine once a day, group C (n = 40) received Tamsulosin 0.4 mg and Tolterodine 4 mg once a day and group D (n = 40) as a control group, received placebo once a day. -

Phenazopyridine | Memorial Sloan Kettering Cancer Center
PATIENT & CAREGIVER EDUCATION Phenazopyridine This information from Lexicomp® explains what you need to know about this medication, including what it’s used for, how to take it, its side effects, and when to call your healthcare provider. Brand Names: US AZO Urinary Pain Relief [OTC]; Baridium [OTC] [DSC]; Pyridium; Urinary Pain Relief [OTC] Brand Names: Canada Phenazo; Pyridium What is this drug used for? It is used to ease pain from a bladder infection. It is used to treat signs of urinary problems. What do I need to tell my doctor BEFORE I take this drug? If you are allergic to this drug; any part of this drug; or any other drugs, foods, or substances. Tell your doctor about the allergy and what signs you had. If you have any of these health problems: Kidney disease or liver disease. This is not a list of all drugs or health problems that interact with this drug. Tell your doctor and pharmacist about all of your drugs (prescription or OTC, natural products, vitamins) and health problems. You must check to make sure that it is safe for you to take this drug with all of your drugs and health problems. Phenazopyridine 1/5 Do not start, stop, or change the dose of any drug without checking with your doctor. What are some things I need to know or do while I take this drug? Tell all of your health care providers that you take this drug. This includes your doctors, nurses, pharmacists, and dentists. This drug is not to be used instead of an antibiotic. -

January 2021 Update
PUBLISHED JULY 08, 2021 OCTOBER/DECEMBER 2020; JANUARY 2021 UPDATE CHANGES TO THE HIGHMARK DRUG FORMULARIES Following is the update to the Highmark Drug Formularies and pharmaceutical management procedures for January 2021. The formularies and pharmaceutical management procedures are updated on a bimonthly basis, and the following changes reflect the decisions made in October, December, and January by our Pharmacy and Therapeutics Committee. These updates are effective on the dates noted throughout this document. Please reference the guide below to navigate this communication: Section I. Highmark Commercial and Healthcare Reform Formularies A. Changes to the Highmark Comprehensive Formulary and the Highmark Comprehensive Healthcare Reform Formulary B. Changes to the Highmark Healthcare Reform Essential Formulary C. Changes to the Highmark Core Formulary D. Changes to the Highmark National Select Formulary E. Updates to the Pharmacy Utilization Management Programs 1. Prior Authorization Program 2. Managed Prescription Drug Coverage (MRxC) Program 3. Formulary Program 4. Quantity Level Limit (QLL) Programs As an added convenience, you can also search our drug formularies and view utilization management policies on the Provider Resource Center (accessible via NaviNet® or our website). Click the Pharmacy Program/Formularies link from the menu on the left. Highmark Blue Cross Blue Shield Delaware is an independent licensee of the Blue Cross and Blue Shield Association. NaviNet is a registered trademark of NaviNet, Inc., which is an independent company that provides secure, web-based portal between providers and health insurance companies. IMPORTANT DRUG SAFETY UPDATES 03/31/2021 – Studies show increased risk of heart rhythm problems with seizure and mental health medicine lamotrigine (Lamictal) in patients with heart disease. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Hormone Replacement Therapy and Osteoporosis
This report may be used, in whole or in part, as the basis for development of clinical practice guidelines and other quality enhancement tools, or a basis for reimbursement and coverage policies. AHRQ or U.S. Department of Health and Human Services endorsement of such derivative products may not be stated or implied. AHRQ is the lead Federal agency charged with supporting research designed to improve the quality of health care, reduce its cost, address patient safety and medical errors, and broaden access to essential services. AHRQ sponsors and conducts research that provides evidence-based information on health care outcomes; quality; and cost, use, and access. The information helps health care decisionmakers— patients and clinicians, health system leaders, and policymakers—make more informed decisions and improve the quality of health care services. Systematic Evidence Review Number 12 Hormone Replacement Therapy and Osteoporosis Prepared for: Agency for Healthcare Research and Quality U.S. Department of Health and Human Services 2101 East Jefferson Street Rockville, MD 20852 http://www.ahrq.gov Contract No. 290-97-0018 Task Order No. 2 Technical Support of the U.S. Preventive Services Task Force Prepared by: Oregon Health Sciences University Evidence-based Practice Center, Portland, Oregon Heidi D. Nelson, MD, MPH August 2002 Preface The Agency for Healthcare Research and Quality (AHRQ) sponsors the development of Systematic Evidence Reviews (SERs) through its Evidence-based Practice Program. With guidance from the third U.S. Preventive Services Task Force∗ (USPSTF) and input from Federal partners and primary care specialty societies, two Evidence-based Practice Centers—one at the Oregon Health Sciences University and the other at Research Triangle Institute-University of North Carolina—systematically review the evidence of the effectiveness of a wide range of clinical preventive services, including screening, counseling, immunizations, and chemoprevention, in the primary care setting. -

Drug Formulary
University of Arkansas March 2017 Use of generic drugs can save both you and your health plan money. This list is not all-inclusive and is not a guarantee of coverage. Plan Benefit design is the final determinate of coverage. Certain drugs (*) may be subject to Prior Authorization (PA), Quantity Limits (QL), Step Therapy (ST), or Reference Based Pricing (RBP) requirements according to Benefit Design. Unless noted, multisource brand drugs (brand drugs with generic equivalent) are covered at 100% copay. If you have any questions about these requirements or other formulary questions, please contact a MedImpact Healthcare customer service representative at 800-788-2949. This list represents brand products in CAPS, branded generics in upper- and lowercase Italics, and generic products in lowercase italics. Drug Type Tier 1 Tier 2 Tier 3 Anti-Infectives Antibiotics – Cephalosporins cefaclor, cefadroxil, cefdinir, CEFTIN susp, SUPRAX 400mg only* (Quantity Limit) cefpodoxime, cefprozil, (QL) cefditoren,cefuroxime, Note: all other Suprax strengths are 100% cephalexin copay Antibiotics - Macrolides azithromycin, clarithromycin, ERY-TAB, ZMAX susp clarithromycin ext-rel, PCE erythromycin delayed-rel, erythromycin ethylsuccinate, erythromycin stearate Antibiotics - Fluoroquinolones ciprofloxacin, ciprofloxacin ext- FACTIVE rel, levofloxacin ,moxifloxacin Antibiotics - Penicillins amoxicillin, amoxicillin- clavulanate, dicloxacillin, penicillin VK Antibiotics – Other* (Prior clindamycin HCl, doxycycline ZYVOX susp*(PA) Authorization) hyclate, linezolid* -

Treatment Approaches for Interstitial Cystitis: Multimodality Therapy Robert J
CLINICAL MANAGEMENT OF INTERSTITIAL CYSTITIS Treatment Approaches for Interstitial Cystitis: Multimodality Therapy Robert J. Evans, MD Moses Cone Health System, Greensboro, NC Interstitial cystitis is an increasingly common disease characterized by urgency, frequency, and pelvic pain. Its etiology is poorly understood but is likely to be multifactorial. A proposed pathophysiology describing a cascade of events, including epithelial dysfunction, mast cell activation, and neurogenic inflam- mation, is presented. Using this model, multimodality therapy regimens have been developed that treat all components of this cascade. Multimodality therapy appears more effective than single agents in the treatment of interstitial cystitis. [Rev Urol. 2002;4(suppl 1):S16–S20] © 2002 MedReviews, LLC Key words: Interstitial cystitis • Multimodality therapy • Pentosan polysulfate • Hydroxyzine hydrochloride • Amitriptyline hydrochloride nterstitial cystitis (IC) is a bladder condition that presents with a range of symptoms, including bladder pain, urinary urgency and frequency, and noc- turia. Current estimates of disease prevalence suggest that at least 1 million I 1 people in the United States are affected. The etiology remains uncertain, although a number of potential causal factors have been proposed.2–7 The variation seen in both the range of symptoms and in patients’ responses to therapy suggest that multiple factors are involved in this disease process. There remains the possibility that subgroups of patients may exist with differing etiologies. S16 VOL. 4 SUPPL. 1 2002 REVIEWS IN UROLOGY Multimodality Therapy for IC demonstrated that IC patients suffer Bladder insult from a defective GAG layer that allows urinary metabolites such as potassium to pass through the bladder wall and into the submucosal space.10 More injury Epithelial layer damage Potassium leaking through the uroepithelium causes depolarization of smooth muscles of the bladder and the pelvis, with activation of Mast cell activation and Potassium leak into sensory nerves. -

Pharmacotherapy for Interstitial Cystitis/Bladder Pain Syndrome
Current Bladder Dysfunction Reports (2019) 14:365–376 https://doi.org/10.1007/s11884-019-00540-9 PHARMACOTHERAPIES AND DRUG DEVELOPMENT/AGENTS (ES ROVNER, SECTION EDITOR) Pharmacotherapy for Interstitial Cystitis/Bladder Pain Syndrome Alyssa Greiman1 & Lindsey Cox 1 Published online: 6 November 2019 # Springer Science+Business Media, LLC, part of Springer Nature 2019 Abstract Purpose of Review Current literature regarding pharmacotherapy treatment strategies available for the management of interstitial cystitis/bladder pain syndrome (IC/BPS) will be addressed including oral, transdermal, and intravesical therapies. Pharmacotherapies with emerging data will be addressed, but the focus is on those treatments described by the AUA guidelines for IC/BPS. Recent Findings While multiple pharmacotherapy options for the management of IC/BPS exist, the evidence for most medical therapies is not strong and frequently yields mixed results. It has been over two decades since a new medication has gained FDA approval for the treatment of IC/BPS. This has prompted clinicians to reassess the approach to evaluating patients with IC/BPS, leading to the advent of phenotype-directed multimodal therapy. Summary Though national and international guidelines recommend a step-wise treatment algorithm beginning with the most conservative treatment options, the evidence for most therapies is mixed. Furthermore, recent randomized controlled trials of promising treatment options have yielded negative results, highlighting the importance of phenotype-directed classification to aid in the current management of IC/BPS and to allow for better research trial designs. Keywords Interstitial cystitis . Bladder pain syndrome . Pentosan polysulfate . Pharmacotherapy Introduction of infection or other identifiable causes.” [1]Thisisthe definition used by the American Urological Association Much of the difficulty surrounding treatment of interstitial (AUA),andassuch,isthedefinitionusedinthescope cystitis or bladder pain syndrome (IC/BPS) centers on the of this review. -

Download ROCKS Patient Education Packet
Paent Educaon Checklist Following Kidney Stone Surgery The purpose of this document is to ensure a standardized method for covering all relevant paent educaon material following kidney stone surgery. It is designed to be used as a checklist while covering the key points within the accompanying ROCKS educaonal resources: Managing Pain and Urinary Symptoms following URS, Ureteral Stents: What to expect and how to manage. Managing Pain and Urinary Symptoms following Ureteroscopy Common symptoms When to call your doctor Medicaons prescribed to manage pain and reduce symptoms (please circle): Alpha‐Blockers Ancholinergics NSAIDs Opioids Other Tamsulosin (Flomax) Oxybutynin (Ditropan) Ketorolac (Toradol) Hydrocodone/acetaminophen Phenazopyridine (Pyridium) (Norco, Vicodin) Alfuzosin (Uroxatral) Tolterodine (Detrol) Ibuprofen (Motrin) Oxycodone/Paracetamol Acetaminophen (Tylenol) (Oxyconn) Other: ___________ Other:___________ Other: ___________ Other: ________________ Other: ________________ Ureteral Stent: What to expect and how to manage (See direcons for viewing our Stent Educaon Video on page 5) Was a stent placed during surgery? Yes No (If yes, complete checklist below) Defining a stent Managing stent‐related symptoms Symptoms associated with a stent Affect on daily acvies STENT REMOVAL My stent will be removed in ________ days Was the stent placed on a string? YES (circle selected opon below) NO (circle selected opon below) Paent removal | Office removal by RN Office removal | Surgical removal What can I expect aer the stent is removed? Page 1 of 5 Managing Pain and Urinary Symptoms following Ureteroscopy • You had surgery to remove or fragment your kidney stones, also known as an ureteroscopy. • After surgery, you may have some degree of pain or discomfort.