The Rabbits Are Prepared ..." - the Development of Ethinylestradiol and Ethinyltestosterone Frobenius W J
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Download Product Insert (PDF)
PRODUCT INFORMATION Quinestrol Item No. 10006320 CAS Registry No.: 152-43-2 Formal Name: 3-(cyclopentyloxy)-19-norpregna- 1,3,5(10)-trien-20-yn-17α-ol Synonyms: Ethylnyl Estradiol-3-cyclopentyl ether, W 3566 MF: C25H32O2 FW: 3645 Purity: ≥98% UV/Vis.: λmax: 202, 281 nm Supplied as: A crystalline solid Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Quinestrol is supplied as a crystalline solid. A stock solution may be made by dissolving the quinestrol in an organic solvent purged with an inert gas. Quinestrol is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF). The solubility of quinestrol in ethanol is approximately 20 mg/ml and approximately 30 mg/ml in DMSO and DMF. Quinestrol is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, should first be dissolved in DMSO and then diluted with the aqueous buffer of choice. Quinestrol has a solubility of approximately 0.5 mg/ml in a 1:1 solution of DMSO:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. Description Quinestrol is a synthetic estrogen that is effective in hormone replacement therapy.1,2 It is a 3-cyclopentyl ether of ethynyl estradiol. After gastrointestinal absorption, it is stored in adipose tissue, where it is slowly released and metabolized in the liver to its active form, ethynyl estradiol. Quinestrol has found limited use in suppressing lactation in postpartum women and, in combination with synthetic progestogens, as contraceptive therapy, although additional studies are needed for both applications. -
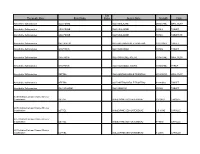
Therapeutic Class Brand Name P a Status Generic
P A Therapeutic Class Brand Name Status Generic Name Strength Form Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 250MG/5ML ORAL SUSP Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET Absorbable Sulfonamides AZULFIDINE SULFASALAZINE 500MG TABLET DR Absorbable Sulfonamides BACTRIM DS SULFAMETHOXAZOLE/TRIMETHO 800-160MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE 500MG TABLET Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML ORAL SUSP Absorbable Sulfonamides GANTRISIN SULFISOXAZOLE ACETYL 500MG/5ML SYRUP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 200-40MG/5 ORAL SUSP Absorbable Sulfonamides SEPTRA SULFAMETHOXAZOLE/TRIMETHO 400-80MG TABLET Absorbable Sulfonamides SULFADIAZINE SULFADIAZINE 500MG TABLET ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-20MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 2.5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-10MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-20MG CAPSULE P A Therapeutic Class Brand Name Status Generic Name Strength Form ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 5-40MG CAPSULE ACE Inhibitor/Calcium Channel Blocker Combination LOTREL AMLODIPINE BESYLATE/BENAZ 10-40MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 10MG CAPSULE Acne Agents, Systemic ACCUTANE ISOTRETINOIN 20MG CAPSULE Acne Agents, Systemic ACCUTANE -

Postmenopausal Pharmacotherapy Newsletter
POSTMENOPAUSAL PHARMACOTHERAPY September, 1999 As Canada's baby boomers age, more and more women will face the option of Hormone Replacement Therapy (HRT). The HIGHLIGHTS decision can be a difficult one given the conflicting pros and cons. M This RxFiles examines the role and use of HRT, as well as newer Long term HRT carries several major benefits but also risks SERMS and bisphosphonates in post-menopausal (PM) patients. which should be evaluated on an individual and ongoing basis MContinuous ERT is appropriate for women without a uterus HRT MWomen with a uterus should receive progestagen (at least 12 HRT is indicated for the treatment of PM symptoms such as days per month or continuous low-dose) as part of their HRT vasomotor disturbances and urogenital atrophy, and is considered MLow-dose ERT (CEE 0.3mg) + Ca++ appears to prevent PMO primary therapy for prevention and treatment of postmenopausal MBisphosphinates (e.g. alendronate, etidronate) and raloxifene are osteoporosis (PMO).1 Contraindications are reviewed in Table 2. alternatives to HRT in treating and preventing PMO Although HRT is contraindicated in women with active breast or M"Natural" HRT regimens can be compounded but data is lacking uterine cancer, note that a prior or positive family history of these does not necessarily preclude women from receiving HRT.1 Comparative Safety: Because of differences between products, some side effects may be alleviated by switching from one product Estrogen Replacement Therapy (ERT) 2 to another, particularly from equine to plant sources or from oral to Naturally secreted estrogens include: topical (see Table 3 - Side Effects & Their Management). -

Clomifene Citrate(BANM, Rinnm) ⊗
2086 Sex Hormones and their Modulators Profasi; UK: Choragon; Ovitrelle; Pregnyl; USA: Chorex†; Choron; Gonic; who received the drug for a shorter period.6 No association be- 8. Werler MM, et al. Ovulation induction and risk of neural tube Novarel; Ovidrel; Pregnyl; Profasi; Venez.: Ovidrel; Pregnyl; Profasi†. tween gonadotrophin therapy and ovarian cancer was noted in defects. Lancet 1994; 344: 445–6. Multi-ingredient: Ger.: NeyNormin N (Revitorgan-Dilutionen N Nr this study. The conclusions of this study were only tentative, 9. Greenland S, Ackerman DL. Clomiphene citrate and neural tube 65)†; Mex.: Gonakor. defects: a pooled analysis of controlled epidemiologic studies since the numbers who developed ovarian cancer were small; it and recommendations for future studies. Fertil Steril 1995; 64: has been pointed out that a successfully achieved pregnancy may 936–41. reduce the risk of some other cancers, and that the risks and ben- 10. Whiteman D, et al. Reproductive factors, subfertility, and risk efits of the procedure are not easy to balance.7 A review8 of epi- of neural tube defects: a case-control study based on the Oxford Clomifene Citrate (BANM, rINNM) ⊗ Record Linkage Study Register. Am J Epidemiol 2000; 152: demiological and cohort studies concluded that clomifene was 823–8. Chloramiphene Citrate; Citrato de clomifeno; Clomifène, citrate not associated with any increase in the risk of ovarian cancer 11. Sørensen HT, et al. Use of clomifene during early pregnancy de; Clomifeni citras; Clomiphene Citrate (USAN); Klomifeenisi- when used for less than 12 cycles, but noted conflicting results, and risk of hypospadias: population based case-control study. -

Differential Effects of Hormones on Cellular Metabolism in Keratoconus
www.nature.com/scientificreports OPEN Differential Effects of Hormones on Cellular Metabolism in Keratoconus In Vitro Received: 27 October 2016 Tina B McKay1, Jesper Hjortdal2, Henrik Sejersen2 & Dimitrios Karamichos1,3 Accepted: 18 January 2017 Keratoconus (KC) is a corneal thinning disease with an onset commonly immediately post-puberty Published: 17 February 2017 and stabilization by 40 to 50 years of age. The role of hormones in regulating corneal tissue structure in homeostatic and pathological conditions is unknown. Our group recently linked altered hormone levels to KC. Our current study sought to investigate and delineate the effects of exogenous hormones, such as androgen, luteotropin, and estrogen, on corneal stroma bioenergetics. We utilized our established 3D in vitro model to characterize the effects of DHEA, prolactin, 17β-estradiol on insulin- growth factor-1 and -2 (IGF-1, -2) signaling and metabolic function in primary corneal fibroblasts from healthy controls (HCFs) and KC patients (HKCs). Our data showed that exogenous DHEA significantly downregulated IGF-1 and its receptor in both HCFs and HKCs with HKCs showing consistently lower basal pentose phosphate flux. Prolactin caused no significant change in IGF-1 levels and an increase in IGF-2 in HKCs correlating with an increase in ATP and NADH levels. 17β-estradiol led to a significant upregulation in pentose phosphate flux and glycolytic intermediates in HCFs. Our results identified hormone-specific responses regulated in HKCs compared to HCFs revealing a novel role for hormones on bioenergetics in KC. Sex hormones play a functional role in regulating growth and reproduction, systemic metabolism, and cellular differentiation and functionality1–3. -

Mass Spectrometry Analysis of Hormones in Water by Direct Injection
Application Note Environmental Mass Spectrometry Analysis of Hormones in Water by Direct Injection Using the Agilent 6470 Triple Quadrupole Mass Spectrometer Authors Abstract Imma Ferrer, E. Michael Thurman, and Some hormones are included in the Contaminant Candidate List CCL4 of the Jerry A. Zweigenbaum Environmental Protection Agency (EPA) to be assessed for regulation in drinking University of Colorado and water. These compounds are also of regulatory interest in the EU, China, and other Agilent Technologies, Inc. countries. Therefore, the environmental community often desires the analysis of these compounds in water samples. This Application Note describes the methodology used for the determination of eight hormones (17-α-ethinylestradiol, 17-β-estradiol, estriol, 4-androstene-3,17-dione, equilin, estrone, progesterone, and testosterone) in tap water using an Agilent 6470 triple quadrupole mass spectrometer. A direct injection method using 100 µL of water sample was carried out. This method saves time, reduces handling errors and analytical variability, and is sensitive enough to detect hormones in surface and drinking water at ng/L levels. Introduction Both positive and negative ion modes and stored at −18 °C. A mix of all were used, depending on the specific the hormones was prepared at a The presence of hormones in compound. We also used a novel concentration of 1 μg/mL. Serial dilutions environmental waters has always been mobile phase approach that included were prepared to obtain a calibration controversial because either none have ammonium fluoride to enhance the curve ranging from 1 to 500 ng/L. been detected, or very low traces have negative ion signal4. -

Chemical Compounds As Carcinogenic Agents Second Supplementary Report: Literature of 1938 and 1939 Biological Considerations
CHEMICAL COMPOUNDS AS CARCINOGENIC AGENTS SECONDSUPPLEMENTARY REPORT: LITERATURE OF 1938 AND 1939 J. W. COOK AND E. L. KENNAWAY (From the Royal Cancer Hospital (Free), London, and the University of Glasgow) BIOLOGICALCONSIDERATIONS (Continued from page 428, Jitly 1940) II. Action of Carcinogenic Compounds in Difierent Species and Tissues A number of reports of the action of carcinogenic compounds on human tis- sues have appeared. Klar (601) developed a nodule on the forearm after completion of a series of experiments with 3:4-benzpyrene. He applied a solution of the hydrocarbon (0.25 per cent in benzol) to the skin of mice with a paint brush and for at least part of the period wore rubber gloves. He also conducted experiments, of which no description is given, with the powdered hydrocarbon contained in a glass vessel. Three months after the completion of the experiments a small nodule appeared on the dorsum of the left forearm, This was excised in May 1938 and described by Professor Huckel as a “ so- called benign calcifying epithelioma.” The growth extended into the subcu- taneous fatty tissue; the connection with the superficial epithelium is not described nor is it evident in the two photomicrographs which illustrate the report. The author does not state his age. Gordonoff and Walthard (562) record the occurrence of a tumor in a labo- ratory assistant, aged forty-two, engaged in applying methylcholanthrene (0.3 per cent in benzol) to the skin of mice. The site was in the nasolabial fold, at a spot often touched by the patient when smoking. The microscopic ap- pearance was that of a “ still well delimited stage of an incipient squamous- cell sarcoma.” Cottini and Mazzone (479) deliberately applied 3 :4-benzpyrene ( 1 per cent in benzene) to the skin, generally of the arm or thigh, of 26 patients with various cutaneous diseases, usually daily for periods up to 120 days. -

The Reactivity of Human and Equine Estrogen Quinones Towards Purine Nucleosides
S S symmetry Article The Reactivity of Human and Equine Estrogen Quinones towards Purine Nucleosides Zsolt Benedek †, Peter Girnt † and Julianna Olah * Department of Inorganic and Analytical Chemistry, Budapest University of Technology and Economics, Szent Gellért tér 4, H-1111 Budapest, Hungary; [email protected] (Z.B.); [email protected] (P.G.) * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Conjugated estrogen medicines, which are produced from the urine of pregnant mares for the purpose of menopausal hormone replacement therapy (HRT), contain the sulfate conjugates of estrone, equilin, and equilenin in varying proportions. The latter three steroid sex hormones are highly similar in molecular structure as they only differ in the degree of unsaturation of the sterane ring “B”: the cyclohexene ring in estrone (which is naturally present in both humans and horses) is replaced by more symmetrical cyclohexadiene and benzene rings in the horse-specific (“equine”) hormones equilin and equilenin, respectively. Though the structure of ring “B” has only moderate influence on the estrogenic activity desired in HRT, it might still significantly affect the reactivity in potential carcinogenic pathways. In the present theoretical study, we focus on the interaction of estrogen orthoquinones, formed upon metabolic oxidation of estrogens in breast cells with purine nucleosides. This multistep process results in a purine base loss in the DNA chain (depurination) and the formation of a “depurinating adduct” from the quinone and the base. The point mutations induced in this manner are suggested to manifest in breast cancer development in the long run. -

Full Text Pdf
Eastern Biologist No. 4 2015 Protective Effects of Conjugated Equine Estrogens and 17-β Estradiol on Oxidatively Stressed Astrocytes Whitley E. Grimes and Kathleen S. Hughes The Eastern Biologist . ♦ A peer-reviewed journal that publishes original articles focused on the many diverse disciplines of biological research, except for the natural history science disciplines (ISSN 2165-6657 [online]). ♦ Subject areas - The journal welcomes manuscripts based on original research and observations, as well as research summaries and general interest articles. Subject areas include, but are not limited to, biochemistry, biotechnology, cell biology, developmental biology, genetics and genomics, immunology, microbiology, molecular evolution, neurobiology, parasitology, physiology, toxicology, as well as scientific pedagogy. ♦ Offers article-by-article online publication for prompt distribution to a global audience ♦ Offers authors the option of publishing large files such as data tables, audio and video clips, and even PowerPoint presentations as online supplemental files. ♦ Special issues - The Eastern Biologist welcomes proposals for special issues that are based on conference proceedings or on a series of invitational articles. Special issue editors can rely on the publisher’s years of experiences in efficiently handling most details relating to the publication of special issues. ♦ Indexing - As is the case with Eagle Hill's other journals, the Eastern Biologist is expected to be fully indexed in Elsevier, Thomson Reuters, Proquest, EBSCO, Google Scholar, and other databases. ♦ The journal staff is pleased to discuss ideas for manuscripts and to assist during all stages of manuscript preparation. The journal has a mandatory page charge to help defray a portion of the costs of publishing the manuscript. -

Enhanced Solubility and Dissolution Rate of Raloxifene Using Cycloencapsulation Technique
Journal of Analytical & Pharmaceutical Research Enhanced Solubility and Dissolution Rate of Raloxifene using Cycloencapsulation Technique Research Article Abstract Volume 2 Issue 5 - 2016 The aim of this study was to improve the water solubility of raloxifene by aqueous solution and solid state was evaluated by the phase solubility diagram, powdercomplexing X-ray it diffractometer,with sulphobutylether-β-cyclodextrin. Fourier-transform infrared Inclusion spectroscopy, complexation nuclear in magnetic resonance, scanning electron microscopy, hot stage microscopy and transmission electron microscopy. The inclusion complex behavior of raloxifene 1Department of Pharmaceutical Technology (Formulation), National Institute of Pharmaceutical Education and Research, classifiedand sulphobutylether-β-cyclodextrin as AL-type curve, indicating the were formation examined of 1:1 by stochiomatric molecular modelinginclusion India method. The phase solubility diagram with sulphobutylether-β-cyclodextrin was complex. The apparent solubility constants calculated from phase solubility 2 -1 Technology Development Center, National Institute of diagram was 753 M . Aqueous solubility and dissolution studies indicated that Pharmaceutical Education and Research, India the dissolution rates were remarkably increased in inclusion complex, compared 3Department of Pharmacoinformatics, National Institute of with the physical mixture and drug alone. In conclusion, inclusion complexation Pharmaceutical Education and Research, India 4 solubility and dissolution rate -

Pp375-430-Annex 1.Qxd
ANNEX 1 CHEMICAL AND PHYSICAL DATA ON COMPOUNDS USED IN COMBINED ESTROGEN–PROGESTOGEN CONTRACEPTIVES AND HORMONAL MENOPAUSAL THERAPY Annex 1 describes the chemical and physical data, technical products, trends in produc- tion by region and uses of estrogens and progestogens in combined estrogen–progestogen contraceptives and hormonal menopausal therapy. Estrogens and progestogens are listed separately in alphabetical order. Trade names for these compounds alone and in combination are given in Annexes 2–4. Sales are listed according to the regions designated by WHO. These are: Africa: Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoros, Congo, Côte d'Ivoire, Democratic Republic of the Congo, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, South Africa, Swaziland, Togo, Uganda, United Republic of Tanzania, Zambia and Zimbabwe America (North): Canada, Central America (Antigua and Barbuda, Bahamas, Barbados, Belize, Costa Rica, Cuba, Dominica, El Salvador, Grenada, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Puerto Rico, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago), United States of America America (South): Argentina, Bolivia, Brazil, Chile, Colombia, Dominican Republic, Ecuador, Guyana, Paraguay, -

Exposure to Female Hormone Drugs During Pregnancy
British Journal of Cancer (1999) 80(7), 1092–1097 © 1999 Cancer Research Campaign Article no. bjoc.1998.0469 Exposure to female hormone drugs during pregnancy: effect on malformations and cancer E Hemminki, M Gissler and H Toukomaa National Research and Development Centre for Welfare and Health, Health Services Research Unit, PO Box 220, 00531 Helsinki, Finland Summary This study aimed to investigate whether the use of female sex hormone drugs during pregnancy is a risk factor for subsequent breast and other oestrogen-dependent cancers among mothers and their children and for genital malformations in the children. A retrospective cohort of 2052 hormone-drug exposed mothers, 2038 control mothers and their 4130 infants was collected from maternity centres in Helsinki from 1954 to 1963. Cancer cases were searched for in national registers through record linkage. Exposures were examined by the type of the drug (oestrogen, progestin only) and by timing (early in pregnancy, only late in pregnancy). There were no statistically significant differences between the groups with regard to mothers’ cancer, either in total or in specified hormone-dependent cancers. The total number of malformations recorded, as well as malformations of the genitals in male infants, were higher among exposed children. The number of cancers among the offspring was small and none of the differences between groups were statistically significant. The study supports the hypothesis that oestrogen or progestin drug therapy during pregnancy causes malformations among children who were exposed in utero but does not support the hypothesis that it causes cancer later in life in the mother; the power to study cancers in offspring, however, was very low.