Progenitor/Stem Cells in Vascular Remodeling During Pulmonary Arterial Hypertension
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hypertension and Coronary Heart Disease
Journal of Human Hypertension (2002) 16 (Suppl 1), S61–S63 2002 Nature Publishing Group All rights reserved 0950-9240/02 $25.00 www.nature.com/jhh Hypertension and coronary heart disease E Escobar University of Chile, Santiago, Chile The association of hypertension and coronary heart atherosclerosis, damage of arterial territories other than disease is a frequent one. There are several patho- the coronary one, and of the extension and severity of physiologic mechanisms which link both diseases. coronary artery involvement. It is important to empha- Hypertension induces endothelial dysfunction, exacer- sise that complications and mortality of patients suffer- bates the atherosclerotic process and it contributes to ing a myocardial infarction are greater in hypertensive make the atherosclerotic plaque more unstable. Left patients. Treatment should be aimed to achieve optimal ventricular hypertrophy, which is the usual complication values of blood pressure, and all the strategies to treat of hypertension, promotes a decrease of ‘coronary coronary heart disease should be considered on an indi- reserve’ and increases myocardial oxygen demand, vidual basis. both mechanisms contributing to myocardial ischaemia. Journal of Human Hypertension (2002) 16 (Suppl 1), S61– From a clinical point of view hypertensive patients S63. DOI: 10.1038/sj/jhh/1001345 should have a complete evaluation of risk factors for Keywords: hypertension; hypertrophy; coronary heart disease There is a strong and frequent association between arterial hypertension.8 Hypertension is frequently arterial hypertension and coronary heart disease associated to metabolic disorders, such as insulin (CHD). In the PROCAM study, in men between 40 resistance with hyperinsulinaemia and dyslipidae- and 66 years of age, the prevalence of hypertension mia, which are additional risk factors of atheroscler- in patients who had a myocardial infarction was osis.9 14/1000 men in a follow-up of 4 years. -

Risk Factors in Abdominal Aortic Aneurysm and Aortoiliac Occlusive
OPEN Risk factors in abdominal aortic SUBJECT AREAS: aneurysm and aortoiliac occlusive PHYSICAL EXAMINATION RISK FACTORS disease and differences between them in AORTIC DISEASES LIFESTYLE MODIFICATION the Polish population Joanna Miko ajczyk-Stecyna1, Aleksandra Korcz1, Marcin Gabriel2, Katarzyna Pawlaczyk3, Received Grzegorz Oszkinis2 & Ryszard S omski1,4 1 November 2013 Accepted 1Institute of Human Genetics, Polish Academy of Sciences, Poznan, 60-479, Poland, 2Department of Vascular Surgery, Poznan 18 November 2013 University of Medical Sciences, Poznan, 61-848, Poland, 3Department of Hypertension, Internal Medicine, and Vascular Diseases, Poznan University of Medical Sciences, Poznan, 61-848, Poland, 4Department of Biochemistry and Biotechnology of the Poznan Published University of Life Sciences, Poznan, 60-632, Poland. 18 December 2013 Abdominal aortic aneurysm (AAA) and aortoiliac occlusive disease (AIOD) are multifactorial vascular Correspondence and disorders caused by complex genetic and environmental factors. The purpose of this study was to define risk factors of AAA and AIOD in the Polish population and indicate differences between diseases. requests for materials should be addressed to J.M.-S. he total of 324 patients affected by AAA and 328 patients affected by AIOD was included. Previously (joannastecyna@wp. published population groups were treated as references. AAA and AIOD risk factors among the Polish pl) T population comprised: male gender, advanced age, myocardial infarction, diabetes type II and tobacco smoking. This study allowed defining risk factors of AAA and AIOD in the Polish population and could help to develop diagnosis and prevention. Characteristics of AAA and AIOD subjects carried out according to clinical data described studied disorders as separate diseases in spite of shearing common localization and some risk factors. -
Pvd-Vs-Pad.Pdf
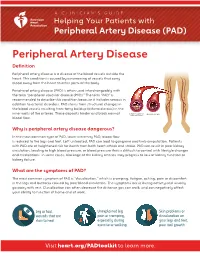
A CLINICIAN'S GUIDE Helping Your Patients with Peripheral Artery Disease (PAD) Peripheral Artery Disease Definition Peripheral artery disease is a disease of the blood vessels outside the heart. This condition is caused by a narrowing of vessels that carry blood away from the heart to other parts of the body. Peripheral artery disease (PAD) is often used interchangeably with the term “peripheral vascular disease (PVD).” The term “PAD” is recommended to describe this condition because it includes venous in addition to arterial disorders. PAD stems from structural changes in the blood vessels resulting from fatty buildup (atherosclerosis) in the inner walls of the arteries. These deposits hinder and block normal blood flow. Why is peripheral artery disease dangerous? In the most common type of PAD, lower extremity PAD, blood flow is reduced to the legs and feet. Left untreated, PAD can lead to gangrene and limb amputation. Patients with PAD are at heightened risk for death from both heart attack and stroke. PAD can result in poor kidney circulation, leading to high blood pressure, or blood pressure that is difficult to control with lifestyle changes and medications. In some cases, blockage of the kidney arteries may progress to loss of kidney function or kidney failure. What are the symptoms of PAD? The most common symptom of PAD is “claudication,” which is cramping, fatigue, aching, pain or discomfort in the legs and buttocks caused by poor blood circulation. The symptoms occur during activity and usually go away with rest. Claudication can often decrease the distance you can walk, and can negatively affect your ability to function at home and at work. -

Atherosclerosisatherosclerosis
AtherosclerosisAtherosclerosis Atherosclerotic Cardiovascular Disease (ASCVD) Smooth m. proliferation Endothelial injury Lipids (cholesterol) Pathogenesis of atherosclerosis 1 Normal Artery Structure Lipoprotein particle 2 XX 60,00060,000 xx 180,000180,000 Robert Hamilton, Ph.D. EM: Negative staining Cardiovascular Research Inst., UCSF 3 The cholesterol in LDL accounts for ©Medscape approx. 70% of the plasma cholesterol Arteriosclerosis (Hardening of the arteries) Arterial wall thickening + loss of elasticity Monckeberg medial Arteriolosclerosis Atherosclerosis calcific sclerosis hyaline hyper- plastic ¾Age 50 -small arteries/arterioles -aorta & branches + ¾Radiologic calcif. -hyaline type / hyperplastic coronary arteries ¾Lumen intact -hypertension / diabetes -ASCVD causes 38% of ¾Clinically insignif. all deaths in N. America 4 ATHEROSCLEROSIS: response-to-injury model Atherosclerosis is a chronic inflammatory response of the arterial wall to endothelial injury. 1. Chronic endothelial injury 2. Accumulation of lipoproteins (LDL mainly) basic tenets 3. Monocyte adhesion to endothelium 4. Platelet adhesion 5. Factors releasedÆSMC recruitment 6. SMC proliferation and ECM production 7. Lipid accumulation: extracellular/mac-SMC Risk Factors for Atherosclerosis •Hyperlipidemia •Smoking •Hypertension •Turbulence •Genetics 5 Endothelial injury Early Chronic—repetitive injury non- denuding endothelial dysfunction -cig. smoke toxins -homocysteine -?? Infectious agents -cytokinesÆgenes for Endothelial injury Early Chronic—repetitive injury non- -

Atherosclerosis
Atherosclerosis Circulating the Facts About Peripheral Vascular Disease Brought to you by the Education Committee of the Society for Vascular Nursing 1 www.svnnet.org Society for Circulating the Facts About Peripheral Artery Disease: Vascular Atherosclerosis Nursing ATHEROSCLEROSIS ● What is ATHEROSCLEROSIS? ● Signs and symptoms ● Risk factors ● Prevent further disease ● Diagnostic tests What Is Atherosclerosis? Blood flows through tubes called arteries which carry oxygen and food to your body and internal organs. Atherosclerosis is a buildup of cholesterol and fat in the artery and is also known as plaque. Arteries become blocked and narrowed due to atherosclerosis and this affects blood flow to your body. Atherosclerosis can affect any artery in the body, including arteries in the heart, brain, arms, legs, pelvis, and kidneys. Signs and Symptoms Signs and symptoms of atherosclerosis are related to the location and amount of narrowing (plaque) that causes decreased blood flow through arteries. The following arteries can be affected by atherosclerosis: Carotid Arteries Blood flows to your brain through the carotid arteries located on either side of the neck. Plaque that narrows or blocks blood flow in the carotid arteries may cause: ● Sudden body weakness ● Weakness/ unable to move one side of the body ● Sudden imbalance or falling ● Droop on one side of the mouth or face ● Temporary or permanent blindness or loss of the vision in one eye ● Difficulty speaking and understanding words ● Memory loss or sudden confusion ● Loss of consciousness ● Sudden and severe headache ● Difficulty with breathing Updated 09052014 1 Society for Circulating the Facts About Peripheral Artery Disease: Vascular Atherosclerosis Nursing Coronary Arteries Blood flows to your heart through the coronary arteries. -

A Public Health Action Plan to Prevent Heart Disease and Stroke
Heart Disease and Stroke Prevention SECTION 1. HEART DISEASE AND STROKE PREVENTION: TIME FOR ACTION Summary The continuing epidemic of cardiovascular diseases (CVD) in the United States and globally calls for renewed and intensified public health action to prevent heart disease and stroke. Public health agencies at national, state, and local levels (including CDC in partnership with NIH) bear a special responsibility to meet this call, along with tribal organizations and all other interested partners. The widespread occurrence and silent progression of atherosclerosis and high blood pressure (the dominant conditions underlying heart disease and stroke) has created a CVD burden that is massive in terms of its attendant death, disability, and social and economic costs. This burden is projected to increase sharply by 2020 because of the changing age structure of the U.S. population and other factors, including the rising prevalence of obesity and diabetes. Several popular myths and misconceptions have obscured this reality, and these must be dispelled through effective communication with the public at large and with policy makers. More than a half-century of research and experience has provided a strong scientific basis for preventing heart disease and stroke. Policy statements and guidelines for prevention have been available for more than four decades and have increased in breadth, depth, and number to guide both public health action and clinical practice. National public health goals have been updated to 2010 and include a specific call to prevent heart disease and stroke. Achieving this goal would greatly accelerate progress toward achieving the nation’s two overarching health goals—increasing quality and years of healthy life and eliminating health disparities. -

Complication Prevention for Patients with Hypertension a Noncommunicable Disease Education Manual for Primary Health Care Professionals and Patients
Complication prevention for patients with hypertension A noncommunicable disease education manual for primary health care professionals and patients Complication prevention for patients with hypertension A noncommunicable disease education manual for primary health care professionals and patients The Noncommunicable Disease Education Manual for Primary Health Care Professionals and Patients results from the contributions and hard work of many people. Its development was led by Dr Hai-Rim Shin, Coordinator, and Dr Warrick Junsuk Kim, Medical Officer, of the Noncommunicable Diseases and Health Promotion unit at the WHO Regional Office for the Western Pacific (WHO/WPRO/NCD) in Manila, Philippines. WHO graciously acknowledges the intellectual contributions of Dr Jung-jin Cho, Co-director, Community-based Primary Care Project Committee and Professor, Department of Family Medicine, Hallym University Sacred Heart Dongtan Hospital, Republic of Korea; Dr Hyejin Lee, Volunteer, WHO/WPRO/NCD (currently PhD candidate, Department of Family Medicine, Seoul National University, Republic of Korea); Ms Saki Narita, Volunteer, WHO/WPRO/NCD (currently PhD candidate, Department of Global Health Policy, Graduate School of Medicine, University of Tokyo, Japan); and Mr Byung Ki Kwon, Technical Officer, WHO/WPRO/NCD (currently Director, Division of Health Promotion, Ministry of Health and Welfare, Republic of Korea). Many thanks to Dr Albert Domingo, Dr Sonia McCarthy, Ms Marie Clem Carlos, Dr Katrin Engelhardt, Mr Kelvin Khow Chuan Heng and Dr Roberto Andres Ruiz from the WHO Regional Office for the Western Pacific and Dr Ma. Charina Benedicto, Physician-in-Charge, Bagong Barangay Health Center & Lying-in Clinic, Pandacan, Manila, Philippines for reviewing the draft publication. Financial support for this publication was received from the Korea Centers for Disease Control and Prevention, Republic of Korea. -

Hypertension – the Silent Killer
Journal of Pre-Clinical and Clinical Research, 2011, Vol 5, No 2, 43-46 REVIEW www.jpccr.eu Hypertension – The Silent Killer Katarzyna Sawicka1,2, Michał Szczyrek1, Iwona Jastrzębska1, Marek Prasał2, Agnieszka Zwolak1, Jadwiga Daniluk1 1 Chair of Internal Medicine and Department of Internal Medicine in Nursing, Medical University, Lublin, Poland 2 Department of Cardiology, Medical University, Lublin, Poland Abstract Hypertension is often called ‘the silent killer’ because it shows no early symptoms and, simultaneously, is the single most signifi cant risk factor for atherosclerosis and all clinical manifestations of atherosclerosis. It is an independent predisposing factor for heart failure, coronary artery disease, stroke, renal disease, and peripheral arterial disease. It is the most important risk factor for cardiovascular morbidity and mortality in industrialized countries. Key words hypertension, complications of hypertension INTRODUCTION diastolic blood pressure fall into diff erent categories, the highest category is used in assessing total cardiovascular Th e leading cause of mortality, responsible for roughly risk [1, 3]. one-third of all deaths globally, is cardiovascular disease. Th ere are 2 types of hypertension depending on etiology Th e majority of these events are caused not by one single – ‘primary’ (also called ‘essential’) hypertension and cardiovascular risk factor, but rather a mixture of several ‘secondary’ hypertension. Essential hypertension is the most factors. Th e most important of these in industrialized prevalent type, aff ecting 90-95% of hypertensive patients [4]. countries is not only hypertension, but also high levels of Th e pathogenesis of primary hypertension is multifactorial blood lipids, obesity, physical inactivity, smoking, glucose and complicated. -

Peripheral Arterial Disease Serena J
www.aging.arizona.edu June 2017 ELDER CARE A Resource for Interprofessional Providers Peripheral Arterial Disease Serena J. Scott, MD and Barry D Weiss, MD, College of Medicine, University of Arizona Peripheral arterial disease (PAD) of the lower extremity is gait may point to the diagnosis of PAD, but physical a common manifestation of atherosclerosis. It is present in findings have not been found to correlate with the up to 20% of older men and women, and estimated to presence or absence of disease. affect more than 200 million people worldwide. The A more accurate approach is to measure the ankle- major risk factors for PAD are smoking, hypertension, and brachial index (ABI), a simple test that can be performed diabetes. PAD is an important problem in older adults due in routine office practice. ABI should be performed in to its prevalence, its often-subtle symptoms, and its high-risk patients, even if asymptomatic. The proper importance as a marker for widespread vascular disease. method for measuring the ABI is outlined in Table 2 and Patients with asymptomatic PAD are at greater risk for illustrated in Figure 1. Table 3 reviews the interpretation functional decline compared to those without PAD. Most of ABI data. Measuring ABI after exercise (on a treadmill) importantly, identifying and treating PAD can improve an may also have a role. Some studies have found individual’s functional status and quality of life. abnormal post-exercise ABI results in patients with normal The presence of PAD in the lower extremity signifies a high resting ABIs, and such findings can be predictive of the likelihood that atherosclerosis is also present elsewhere – need for future revascularization surgery. -

Primary Pulmonary Arteries Atherosclerosis: Discovering an Unusual Cause of Death in Forensic Practice
Rom J Leg Med [20] 177-180 [2012] DOI: 10.4323/rjlm.2012.177 © 2012 Romanian Society of Legal Medicine Primary pulmonary arteries atherosclerosis: discovering an unusual cause of death in forensic practice Michela Cicconi1, Alessandro Bonsignore1, Giulio Fraternali Orcioni2, Francesco Ventura1* _________________________________________________________________________________________ Abstract: Background: In the literature, there are few studies on atherosclerosis in the pulmonary artery in human beings and no cases similar to the one presented has been reported until now. The aim of the study is to describe a particularly unusual case of primary severe pulmonary atherosclerosis, in a 40-year old man, reporting its pathological aspects associated with interstitial lung disease as a cause of a mild pulmonary fibrosis. Case presentation: The patient had marked atherosclerosis in the pulmonary trunk and its branches, probably caused by a series of hemodynamic and endothelial changes, subsequent to the pulmonary hypertension. An autopsy was performed a few days after death in order to explain the reasons of the sudden death. Despite the typical pattern of pulmonary atherosclerosis is generally associated with many co-morbidities, we have found only a significant right ventricular hypertrophy. A complete forensic approach led to attribute the cause of death to cardiorespiratory failure due to severe pulmonary atherosclerosis. Conclusion: in the light of the limited number of reports in the literature, this paper seeks to widen knowledge in the -

Atherosclerosis and Venous Thromboembolism — Similarities
Kardiologia Polska 2013; 71, 12: 1223–1228; DOI: 10.5603/KP.2013.0322 ISSN 0022–9032 ARTYKUŁ SPECJALNY / STATE-OF-THE-ART REVIEW Atherosclerosis and venous thromboembolism — similarities Anetta Undas Institute of Cardiology, Jagiellonian University Medical College, John Paul II Hospital, Krakow, Poland INTRODUCTION tion of the thrombotic mass in both types of vessels [3, 4]. Venous thromboembolism (VTE), encompass- Despite the key role of activated platelets as a driving factor of ing deep vein thrombosis (DVT) and pulmo- thrombus formation under high shear stress conditions, there is nary embolism (PE), and atherothrombosis convincing evidence for a significant contribution of thrombin have long been considered to be separate and blood coagulation proteins in arterial thrombosis, as well entities with distinct pathogenic mechanisms. as for their expression in macrophages and other cells within The traditional rationale for this con- atherosclerotic lesions [5]. cept has been based on: (1) pathologic data Until the beginning of the current century, there was no showing platelet-rich thrombi in the arteries in contrast to clinical data to support basic science findings indicating that ‘red clots’ observed in veins; (2) clinical data on a substantial thrombus formation in most vessels follows the same pattern efficacy of antiplatelet agents in the prevention of arterial and once deranged haemostasis tilted toward thrombosis thromboembolic events in contrast to negligible benefits results in increased risk of thromboembolic events putting from these drugs in patients with VTE; (3) experimental data in danger both the arterial and venous beds. Within the last demonstrating diffuse inflammatory infiltrates, extracellular decade, accumulating evidence has indicated that patients cholesterol deposits, neovessel formation and calcification following VTE are at risk of MI or ischaemic stroke and vice within advanced atherosclerotic lesions within the arterial versa. -

A Review on the Value of Imaging in Differentiating Between Large Vessel Vasculitis and Atherosclerosis
Journal of Personalized Medicine Review A Review on the Value of Imaging in Differentiating between Large Vessel Vasculitis and Atherosclerosis Pieter H. Nienhuis 1,* , Gijs D. van Praagh 1 , Andor W. J. M. Glaudemans 1, Elisabeth Brouwer 2 and Riemer H. J. A. Slart 1,3 1 Department of Nuclear Medicine and Molecular Imaging, Medical Imaging Center, University of Groningen, University Medical Center Groningen, 9700 RB Groningen, The Netherlands; [email protected] (G.D.v.P.); [email protected] (A.W.J.M.G.); [email protected] (R.H.J.A.S.) 2 Department of Rheumatology and Clinical Immunology, University of Groningen, University Medical Center Groningen, 9700 RB Groningen, The Netherlands; [email protected] 3 Department of Biomedical Photonic Imaging, Faculty of Science and Technology, University of Twente, 7500 AE Enschede, The Netherlands * Correspondence: [email protected] Abstract: Imaging is becoming increasingly important for the diagnosis of large vessel vasculitis (LVV). Atherosclerosis may be difficult to distinguish from LVV on imaging as both are inflammatory conditions of the arterial wall. Differentiating atherosclerosis from LVV is important to enable optimal diagnosis, risk assessment, and tailored treatment at a patient level. This paper reviews the current evidence of ultrasound (US), 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography (FDG-PET), computed tomography (CT), and magnetic resonance imaging (MRI) to distinguish LVV from atherosclerosis. In this review, we identified a total of eight studies comparing LVV patients Citation: Nienhuis, P.H.; van Praagh, to atherosclerosis patients using imaging—four US studies, two FDG-PET studies, and two CT G.D.; Glaudemans, A.W.J.M.; studies.