BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Superfamily Calopterygoidea in South China: Taxonomy and Distribution. Progress Report for 2009 Surveys Zhang Haomiao* *PH D
International Dragonfly Fund - Report 26 (2010): 1-36 1 The Superfamily Calopterygoidea in South China: taxonomy and distribution. Progress Report for 2009 surveys Zhang Haomiao* *PH D student at the Department of Entomology, College of Natural Resources and Environment, South China Agricultural University, Guangzhou 510642, China. Email: [email protected] Introduction Three families in the superfamily Calopterygoidea occur in China, viz. the Calo- pterygidae, Chlorocyphidae and Euphaeidae. They include numerous species that are distributed widely across South China, mainly in streams and upland running waters at moderate altitudes. To date, our knowledge of Chinese spe- cies has remained inadequate: the taxonomy of some genera is unresolved and no attempt has been made to map the distribution of the various species and genera. This project is therefore aimed at providing taxonomic (including on larval morphology), biological, and distributional information on the super- family in South China. In 2009, two series of surveys were conducted to Southwest China-Guizhou and Yunnan Provinces. The two provinces are characterized by karst limestone arranged in steep hills and intermontane basins. The climate is warm and the weather is frequently cloudy and rainy all year. This area is usually regarded as one of biodiversity “hotspot” in China (Xu & Wilkes, 2004). Many interesting species are recorded, the checklist and photos of these sur- veys are reported here. And the progress of the research on the superfamily Calopterygoidea is appended. Methods Odonata were recorded by the specimens collected and identified from pho- tographs. The working team includes only four people, the surveys to South- west China were completed by the author and the photographer, Mr. -

Shop Direct Factory List Dec 18
Factory Factory Address Country Sector FTE No. workers % Male % Female ESSENTIAL CLOTHING LTD Akulichala, Sakashhor, Maddha Para, Kaliakor, Gazipur, Bangladesh BANGLADESH Garments 669 55% 45% NANTONG AIKE GARMENTS COMPANY LTD Group 14, Huanchi Village, Jiangan Town, Rugao City, Jaingsu Province, China CHINA Garments 159 22% 78% DEEKAY KNITWEARS LTD SF No. 229, Karaipudhur, Arulpuram, Palladam Road, Tirupur, 641605, Tamil Nadu, India INDIA Garments 129 57% 43% HD4U No. 8, Yijiang Road, Lianhang Economic Development Zone, Haining CHINA Home Textiles 98 45% 55% AIRSPRUNG BEDS LTD Canal Road, Canal Road Industrial Estate, Trowbridge, Wiltshire, BA14 8RQ, United Kingdom UK Furniture 398 83% 17% ASIAN LEATHERS LIMITED Asian House, E. M. Bypass, Kasba, Kolkata, 700017, India INDIA Accessories 978 77% 23% AMAN KNITTINGS LIMITED Nazimnagar, Hemayetpur, Savar, Dhaka, Bangladesh BANGLADESH Garments 1708 60% 30% V K FASHION LTD formerly STYLEWISE LTD Unit 5, 99 Bridge Road, Leicester, LE5 3LD, United Kingdom UK Garments 51 43% 57% AMAN GRAPHIC & DESIGN LTD. Najim Nagar, Hemayetpur, Savar, Dhaka, Bangladesh BANGLADESH Garments 3260 40% 60% WENZHOU SUNRISE INDUSTRIAL CO., LTD. Floor 2, 1 Building Qiangqiang Group, Shanghui Industrial Zone, Louqiao Street, Ouhai, Wenzhou, Zhejiang Province, China CHINA Accessories 716 58% 42% AMAZING EXPORTS CORPORATION - UNIT I Sf No. 105, Valayankadu, P. Vadugapal Ayam Post, Dharapuram Road, Palladam, 541664, India INDIA Garments 490 53% 47% ANDRA JEWELS LTD 7 Clive Avenue, Hastings, East Sussex, TN35 5LD, United Kingdom UK Accessories 68 CAVENDISH UPHOLSTERY LIMITED Mayfield Mill, Briercliffe Road, Chorley Lancashire PR6 0DA, United Kingdom UK Furniture 33 66% 34% FUZHOU BEST ART & CRAFTS CO., LTD No. 3 Building, Lifu Plastic, Nanshanyang Industrial Zone, Baisha Town, Minhou, Fuzhou, China CHINA Homewares 44 41% 59% HUAHONG HOLDING GROUP No. -
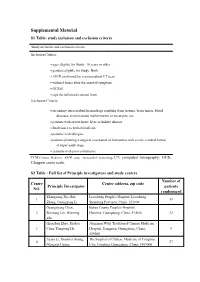
Supplemental Material S1 Table: Study Inclusion and Exclusion Criteria
Supplemental Material S1 Table: study inclusion and exclusion criteria Study inclusion and exclusion criteria Inclusion Criteria: ages eligible for Study: 18 years or older genders eligible for Study: Both AICH confirmed by craniocerebral CT scan within 6 hours after the onset of symptom GCS≥6 sign the informed consent form Exclusion Criteria: secondary intracerebral hemorrhage resulting from trauma, brain tumor, blood diseases, arteriovenous malformation or aneurysm, etc; patients with severe heart, liver or kidney disease. Intolerance to herbal medicine, patients with allergies patients planning a surgical evacuation of hematoma with severe cerebral hernia at super-early stage patients with poor compliance TCM,Chinese Medicine. AICH, acute intracerebral hemorrhage.CT, computed tomography; GCS, Glasgow coma scale. S2 Table : Full list of Principle investigators and study centers Number of Centre Centre address, zip code Principle Investigator patients NO. randomized Zhangyong Xia, Rui Liaocheng People's Hospital, Liaocheng, 1 39 Zhang, Guangzeng Li Shandong Province, China, 252000 Guangsheng Chen, Boluo County People's Hospital, 2 Bochang Lin, Weiming Huizhou, Guangdong, China, 514610 32 Zhu Qianshan Zhao, Richao Jiangmen Wuyi Traditional Chinese Medicine 3 Chen, Yongtong He Hospital, Jiangmen, Guangdong, China, 9 529000 Jiexia Li, Xiaomei Huang, The hospital of Chinese Medicine of Conghua 4 27 Mengxin Huang City, Conghua, Guangdong, China, 5109000 Chaojun Chen, Jianfang Guangzhou Hospital of Integrated traditional 5 Hu, Peiqun -

Factory Address Country
Factory Address Country Durable Plastic Ltd. Mulgaon, Kaligonj, Gazipur, Dhaka Bangladesh Lhotse (BD) Ltd. Plot No. 60&61, Sector -3, Karnaphuli Export Processing Zone, North Potenga, Chittagong Bangladesh Bengal Plastics Ltd. Yearpur, Zirabo Bazar, Savar, Dhaka Bangladesh ASF Sporting Goods Co., Ltd. Km 38.5, National Road No. 3, Thlork Village, Chonrok Commune, Korng Pisey District, Konrrg Pisey, Kampong Speu Cambodia Ningbo Zhongyuan Alljoy Fishing Tackle Co., Ltd. No. 416 Binhai Road, Hangzhou Bay New Zone, Ningbo, Zhejiang China Ningbo Energy Power Tools Co., Ltd. No. 50 Dongbei Road, Dongqiao Industrial Zone, Haishu District, Ningbo, Zhejiang China Junhe Pumps Holding Co., Ltd. Wanzhong Villiage, Jishigang Town, Haishu District, Ningbo, Zhejiang China Skybest Electric Appliance (Suzhou) Co., Ltd. No. 18 Hua Hong Street, Suzhou Industrial Park, Suzhou, Jiangsu China Zhejiang Safun Industrial Co., Ltd. No. 7 Mingyuannan Road, Economic Development Zone, Yongkang, Zhejiang China Zhejiang Dingxin Arts&Crafts Co., Ltd. No. 21 Linxian Road, Baishuiyang Town, Linhai, Zhejiang China Zhejiang Natural Outdoor Goods Inc. Xiacao Village, Pingqiao Town, Tiantai County, Taizhou, Zhejiang China Guangdong Xinbao Electrical Appliances Holdings Co., Ltd. South Zhenghe Road, Leliu Town, Shunde District, Foshan, Guangdong China Yangzhou Juli Sports Articles Co., Ltd. Fudong Village, Xiaoji Town, Jiangdu District, Yangzhou, Jiangsu China Eyarn Lighting Ltd. Yaying Gang, Shixi Village, Shishan Town, Nanhai District, Foshan, Guangdong China Lipan Gift & Lighting Co., Ltd. No. 2 Guliao Road 3, Science Industrial Zone, Tangxia Town, Dongguan, Guangdong China Zhan Jiang Kang Nian Rubber Product Co., Ltd. No. 85 Middle Shen Chuan Road, Zhanjiang, Guangdong China Ansen Electronics Co. Ning Tau Administrative District, Qiao Tau Zhen, Dongguan, Guangdong China Changshu Tongrun Auto Accessory Co., Ltd. -

Factory List
FACTORY LIST Our commitment to transparency is core to our Corporate Social Responsibility efforts. Nordstrom’s Tier 1 factory list for Nordstrom Made, our family of private-label brands, is included below. This list reflects our most strategic suppliers, which we classify internally as Level 1 and Level 2. The data is current as of December 11, 2020. Additional information about our commitment to human rights, including our goals for ethical labor practices and women’s empowerment, can be found on nordstromcares.com. Factory Name Manufacturer Address City State/Province Country Employees Product Type (Male/Female) Industria de Calcados Karlitos Ltda. South Service Trading S/A Rua Benedito Merlino, 999, 14405-448 Franca Sao Paulo Brazil 146 (62/84) Footwear Industria de Calcados Kissol Ltda. South Service Trading S/A Rua Irmãos Antunes, 813, Jardim Franca Sao Paulo Brazil 196 (137/59) Footwear Guanabara, 14405-445 Eminent Garment (Cambodia) Eminent Garment Limited Phum Preak Thmey, Khum Teukvil, Srok Saang Khet Kandal Cambodia 866 (105/761) Woven Limited Saang, Phnom Penh Zhejiang Sunmans Knitting Co. Ltd. Royal Bermuda LLC No. 139 North of Biyun Road, 314400 Haining Zhejiang China 197 (39/158) Accessories West Coast Hosiery Group Dongguan Mayflower Footwear Corp. Pagoda International Footwear Golden Dragon Road, 1st Industrial Park of Dongcheng Dongguan China 500 (350/150) Footwear Sangyuan, 523119 Fujian Fuqing Huatai Footwear Co. Madden Intl. Ltd. Trading Lingjiao Village, Shangjing Town Fuqing Fujian China 360 (170/190) Footwear Ltd. Dolce Vita Intl. Nanyuan Knitting & Garments Co. South Asia Knitting Factory Ltd. Nanhua Industry District, Shengxin Town Nanan City Fujian China 604 (152/452) Sweaters Ltd. -

Clarks Tannery List
LEATHER SUPPLIERS (TANNERIES) SS21 COUNTRY SUPPLIER GROUP FACTORY NAME ADDRESS Argentina Hispano S.A. La Hispano Argentina Curtiembre y charoleria WORKERS <500 Av. Juan B Alberdi 5045/49 FEMALE MALE Buenos Aires OAR ID AR2020028Y07RTR Argentina GEOLOCATION C1440AAB -34.6458547 -58.4964907 Argentina Sadesa Sadesa S.A. WORKERS 501 - 1,000 DE LA RIBERA SUR 664 FEMALE <25% MALE >75% LOMAS DE ZAMORA OAR ID AR20200284WGEGF PCIA DE BS.AIRES REP. ARGENTINA GEOLOCATION -34.7081658 -58.4536824 Brazil CBC Couros e acabamentos Ltda. WORKERS <500 Estrada Leopoldo Petry FEMALE MALE 255 OAR ID BR2020028AGT1C3 Novo Hamburgo RS. Brazil GEOLOCATION -29.716984 -51.1090575 China Nedlink Resources Ltd WORKERS <500 Fu Ze 2nd Street, Fu Ze Road FEMALE <25% MALE >75% Gao Ping Industrial Zone OAR ID CN2020028QXMCE1 San Jiao Zhongshan GEOLOCATION Guangdong, China 528445 22.677198 113.46188 Page 1 of 14 LEATHER SUPPLIERS (TANNERIES) SS21 COUNTRY SUPPLIER GROUP FACTORY NAME ADDRESS China DongGuan JingHong Shoe Material CO.LTD WORKERS <500 South Industrial Zone FEMALE >25% MALE >50% LiuChongWei OAR ID CN20200282REYPV WanJiang District DongGuan City GEOLOCATION GuangDong province 23.047104 113.738543 China Heng Tong Guangzhou Xuna Leather Co.,Ltd. WORKERS <500 2/F,B/D 4 Yili Industrial Park FEMALE >50% MALE >25% Longtang OAR ID CN2020028VNGX95 Qingcheng District Qingyuan GEOLOCATION Guangdong 23.471394 113.0038866 China Heng Tong Anhui Hengtong Leather Co., Ltd. WORKERS <500 Linjiang Industrial Park FEMALE >50% MALE >25% Fuxing Town OAR ID CN2020028TZ7TPF Susong County Anqing GEOLOCATION Anhui 41.811979 126.918087 China Hispano Huizhou Modapelle Leathers Ltd. WORKERS <500 Shang Sha Road, Sha Tou Industrial District, FEMALE >50% MALE >25% Yuan Zhou Town OAR ID CN2020028R1GM62 Boluo County Huizhou GEOLOCATION Guangdong, PRC 23.075215 114.100057 Page 2 of 14 LEATHER SUPPLIERS (TANNERIES) SS21 COUNTRY SUPPLIER GROUP FACTORY NAME ADDRESS China ISA ISA Heshan Trading Limited WORKERS <500 No. -

Levi Strauss & Co. Factory List
Levi Strauss & Co. Factory List Published : November 2019 Total Number of LS&Co. Parent Company Name Employees Country Factory name Alternative Name Address City State Product Type (TOE) Initiatives (Licensee factories are (Workers, Staff, (WWB) blank) Contract Staff) Argentina Accecuer SA Juan Zanella 4656 Caseros Accessories <1000 Capital Argentina Best Sox S.A. Charlone 1446 Federal Apparel <1000 Argentina Estex Argentina S.R.L. Superi, 3530 Caba Apparel <1000 Argentina Gitti SRL Italia 4043 Mar del Plata Apparel <1000 Argentina Manufactura Arrecifes S.A. Ruta Nacional 8, Kilometro 178 Arrecifes Apparel <1000 Argentina Procesadora Serviconf SRL Gobernardor Ramon Castro 4765 Vicente Lopez Apparel <1000 Capital Argentina Spring S.R.L. Darwin, 173 Federal Apparel <1000 Asamblea (101) #536, Villa Lynch Argentina TEXINTER S.A. Texinter S.A. B1672AIB, Buenos Aires Buenos Aires <1000 Argentina Underwear M&S, S.R.L Levalle 449 Avellaneda Apparel <1000 Argentina Vira Offis S.A. Virasoro, 3570 Rosario Apparel <1000 Plot # 246-249, Shiddirgonj, Bangladesh Ananta Apparels Ltd. Nazmul Hoque Narayangonj-1431 Narayangonj Apparel 1000-5000 WWB Ananta KASHPARA, NOYABARI, Bangladesh Ananta Denim Technology Ltd. Mr. Zakaria Habib Tanzil KANCHPUR Narayanganj Apparel 1000-5000 WWB Ananta Ayesha Clothing Company Ltd (Ayesha Bangobandhu Road, Tongabari, Clothing Company Ltd,Hamza Trims Ltd, Gazirchat Alia Madrasha, Ashulia, Bangladesh Hamza Clothing Ltd) Ayesha Clothing Company Ltd( Dhaka Dhaka Apparel 1000-5000 Jamgora, Post Office : Gazirchat Ayesha Clothing Company Ltd (Ayesha Ayesha Clothing Company Ltd(Unit-1)d Alia Madrasha, P.S : Savar, Bangladesh Washing Ltd.) (Ayesha Washing Ltd) Dhaka Dhaka Apparel 1000-5000 Khejur Bagan, Bara Ashulia, Bangladesh Cosmopolitan Industries PVT Ltd CIPL Savar Dhaka Apparel 1000-5000 WWB Epic Designers Ltd 1612, South Salna, Salna Bazar, Bangladesh Cutting Edge Industries Ltd. -

Factory Name
Factory Name Factory Address BANGLADESH Company Name Address AKH ECO APPARELS LTD 495, BALITHA, SHAH BELISHWER, DHAMRAI, DHAKA-1800 AMAN GRAPHICS & DESIGNS LTD NAZIMNAGAR HEMAYETPUR,SAVAR,DHAKA,1340 AMAN KNITTINGS LTD KULASHUR, HEMAYETPUR,SAVAR,DHAKA,BANGLADESH ARRIVAL FASHION LTD BUILDING 1, KOLOMESSOR, BOARD BAZAR,GAZIPUR,DHAKA,1704 BHIS APPARELS LTD 671, DATTA PARA, HOSSAIN MARKET,TONGI,GAZIPUR,1712 BONIAN KNIT FASHION LTD LATIFPUR, SHREEPUR, SARDAGONI,KASHIMPUR,GAZIPUR,1346 BOVS APPARELS LTD BORKAN,1, JAMUR MONIPURMUCHIPARA,DHAKA,1340 HOTAPARA, MIRZAPUR UNION, PS : CASSIOPEA FASHION LTD JOYDEVPUR,MIRZAPUR,GAZIPUR,BANGLADESH CHITTAGONG FASHION SPECIALISED TEXTILES LTD NO 26, ROAD # 04, CHITTAGONG EXPORT PROCESSING ZONE,CHITTAGONG,4223 CORTZ APPARELS LTD (1) - NAWJOR NAWJOR, KADDA BAZAR,GAZIPUR,BANGLADESH ETTADE JEANS LTD A-127-131,135-138,142-145,B-501-503,1670/2091, BUILDING NUMBER 3, WEST BSCIC SHOLASHAHAR, HOSIERY IND. ATURAR ESTATE, DEPOT,CHITTAGONG,4211 SHASAN,FATULLAH, FAKIR APPARELS LTD NARAYANGANJ,DHAKA,1400 HAESONG CORPORATION LTD. UNIT-2 NO, NO HIZAL HATI, BAROI PARA, KALIAKOIR,GAZIPUR,1705 HELA CLOTHING BANGLADESH SECTOR:1, PLOT: 53,54,66,67,CHITTAGONG,BANGLADESH KDS FASHION LTD 253 / 254, NASIRABAD I/A, AMIN JUTE MILLS, BAYEZID, CHITTAGONG,4211 MAJUMDER GARMENTS LTD. 113/1, MUDAFA PASCHIM PARA,TONGI,GAZIPUR,1711 MILLENNIUM TEXTILES (SOUTHERN) LTD PLOTBARA #RANGAMATIA, 29-32, SECTOR ZIRABO, # 3, EXPORT ASHULIA,SAVAR,DHAKA,1341 PROCESSING ZONE, CHITTAGONG- MULTI SHAF LIMITED 4223,CHITTAGONG,BANGLADESH NAFA APPARELS LTD HIJOLHATI, -

Factory Name Address City Zip Code Province Country # of Workers Category Jiangsu Asset Underwear Co., Ltd
Factory name Address City Zip code Province Country # of workers Category Jiangsu Asset Underwear Co., Ltd. No. 6, Wang One Road, Economic Development Zone, Lianshui County Huaian 223001 Jiangsu China <1000 apparel Shen Zhen BP Co., Ltd. 1-5 Floor, B12, Hengfeng Industrial Zone, Hezhou, Xixiang Bao'an Area Shenzhen 518100 Guangdong China <1000 apparel Zhong Shan Kin Tak Garment Factory Ltd. Wan An Industrial District, Ji Dong 1, Xiaolan Town Zhongshan 528400 Guangdong China <1000 apparel Zhongshan Vigor Garment Co., Ltd. Chang Ling Lu, Lan Bian Village, NanLangZhen Zhongshan 528400 Guangdong China <1000 apparel Intimate Fashion Co., Ltd. 140 Moo.5, Phutthamonthon 5 Road, Omnoi Kratumban 74130 Samut Sakhon Thailand <1000 apparel Elite Fame Garment Factory Shang Nan, Yuanzhou Town, Bolou Huizhou City Huizhou City 528400 Guangdong China <1000 apparel DongGuan XuYang Textile Co.,Ltd NO.127,yongmao road ,renzhou village,santian town Dongguan 523999 Guangdong China <1000 fabric Maoming City Jinquan Rubber & Plastics Products Co.,Ltd No.57 Qiongsha Road,ShaYuan Town Dianbai District, Maoming 525028 Guangdong China <1000 elastic Hongda High-Tech Holding Co., Ltd No. 118 Jian She Road Xucun Town Haining 311409 Zhejiang China <1000 fabric Fuzhou Meijiahua Knitting & Textile Co., Ltd Room 1416, Building 16th, Haixibaiyue Town 2nd 18 Duyuan Road Fuzhou 350019 Fujian China <1000 fabric Deqing Taihe Industries Co., Ltd Gantang High & New Technology Development Zone Decheng Town Deqing 526600 Guangdong China <1000 fabric Dongguan City Humen Town Xinghui -

Ceramic Tableware from China List of CNCA‐Certified Ceramicware
Ceramic Tableware from China June 15, 2018 List of CNCA‐Certified Ceramicware Factories, FDA Operational List No. 64 740 Firms Eligible for Consideration Under Terms of MOU Firm Name Address City Province Country Mail Code Previous Name XIAOMASHAN OF TAIHU MOUNTAINS, TONGZHA ANHUI HANSHAN MINSHENG PORCELAIN CO., LTD. TOWN HANSHAN COUNTY ANHUI CHINA 238153 ANHUI QINGHUAFANG FINE BONE PORCELAIN CO., LTD HANSHAN ECONOMIC DEVELOPMENT ZONE ANHUI CHINA 238100 HANSHAN CERAMIC CO., LTD., ANHUI PROVINCE NO.21, DONGXING STREET DONGGUAN TOWN HANSHAN COUNTY ANHUI CHINA 238151 WOYANG HUADU FINEPOTTERY CO., LTD FINEOPOTTERY INDUSTRIAL DISTRICT, SOUTH LIUQIAO, WOSHUANG RD WOYANG CITY ANHUI CHINA 233600 THE LISTED NAME OF THIS FACTORY HAS BEEN CHANGED FROM "SIU‐FUNG CERAMICS (CHONGQING SIU‐CERAMICS) CO., LTD." BASED ON NOTIFICATION FROM CNCA CHONGQING CHN&CHN CERAMICS CO., LTD. CHENJIAWAN, LIJIATUO, BANAN DISTRICT CHONGQING CHINA 400054 RECEIVED BY FDA ON FEBRUARY 8, 2002 CHONGQING KINGWAY CERAMICS CO., LTD. CHEN JIA WAN, LI JIA TUO, BANAN DISTRICT, CHONGQING CHINA 400054 BIDA CERAMICS CO.,LTD NO.69,CHENG TIAN SI GE DEHUA COUNTY FUJIAN CHINA 362500 NONE DATIAN COUNTY BAOFENG PORCELAIN PRODUCTS CO., LTD. YONGDE VILLAGE QITAO TOWN DATIAN COUNTY CHINA 366108 FUJIAN CHINA DATIAN YONGDA ART&CRAFT PRODUCTS CO., LTD. NO.156, XIANGSHAN ROAD, JUNXI TOWN, DATIAN COUNTY FUJIAN 366100 DEHUA KAIYUAN PORCELAIN INDUSTRY CO., LTD NO. 63, DONGHUAN ROAD DEHUA TOWN FUJIAN CHINA 362500 THE LISTED ADDRESS OF THIS FACTORY HAS BEEN CHANGED FROM "MAQIUYANG XUNZHONG XUNZHONG TOWN, DEHUA COUNTY" TO THE NEW EAST SIDE, THE SECOND PERIOD, SHIDUN PROJECT ADDRESS LISTED ABOVE BASED ON NOTIFICATION DEHUA HENGHAN ARTS CO., LTD AREA, XUNZHONG TOWN, DEHUA COUNTY FUJIAN CHINA 362500 FROM THE CNCA AUTHORITY IN SEPTEMBER 2014 DEHUA HONGSHENG CERAMICS CO., LTD. -

Spatial-Temporal Change Analysis of Water Area in Pearl River Delta Based on Remote Sensing Technology
Available online at www.sciencedirect.com Procedia Environmental Sciences 10 ( 2011 ) 2170 – 2175 2011 3rd International Conference on Environmental Science and Information Application Technology (ESIAT 2011) Spatial-temporal Change Analysis of Water Area in Pearl River Delta Based on Remote Sensing Technology LIAN Lian a, CHEN Jianfei* School of Geographical Science, Guangzhou University, Guangzhou, 510006, China [email protected] Abstract This paper investigates the dynamic change of water area in Pearl River Delta during the past 20 years, including river, dike-pond and other water, by comparing spatial-temporal changes with reference to the literatures. We used image classification and object identification analysis of TM in 1990, ETM+ in 1999 and SPOT-5 in 2008 by ENVI and ArcGIS. Total water area increases from 4693.1km2 to 5355.7km2 from 1990 to 1999, but decreases to 4636.2km2 in 2008. River area falls from 1990 to 2008, and dike-pond area keeps rising only before 1999, after which it declines. The decrease of water area is mainly caused by human factors. The rapid expansion of industry after reform and opening-up to the world outside is the primary cause of dike-pond area reducing from 1999 to 2008. © 2011 PublishedPublished by by Elsevier Elsevier Ltd. Ltd. Selection Selection and/or and/or peer-review peer-review under under responsibility responsibility of Conference of [name organizer]ESIAT2011 Organization Committee. Keywords: Water Area, Spatial-temporal Change, Remote Sensing, Dike-pond, Pearl River Delta Introduction Water area is one important type of land use/land cover. Water areas such as rivers, dike-ponds are part of the wetland, which are important natural subsystems that possess many ecological and social service functions. -

Regional Division and Statistics in China
Regional Division and Statistics in China Peng, Zhang National Bureau of Statistics of China, 57 Yuetan Nanjie, Xichengqu, Beijing China. E-mail: [email protected] I. Regional Division of China Regional division is the base of researching regional economy, the precondition for exploring regional Sessions development, and the foundation for formulating regional policies. As China is a country with vast territory, Posters there is great difference in the development condition and developmental level among different regions. Therefore, regional division is an important way for China, a country with relatively vast territory, to adjust and control the regional economic development. Thus a complete set of regional division system is an important support for setting down and executing regional policies of China. Regional division includes three aspects: (1) natural division; (2) administrative division; (3) economic regional division. Natural region, administrative region and economic region are three physiographical regional concepts with different nature but close relationship. Natural region is a geographic unit with relatively consistent natural features and ambiguous boundary. Administrative region, belonging to superstructure, is the administrative jurisdiction scope of governments at all levels, and is the region divided as per systems, so it has clear legal boundary line. Economic region is the region strategically divided as per the feature of social production geographic division. As it belongs to economic activity region, the boundary line may change with the Sessions development of economy. Posters If administrative division is largely consistent with economic regional division, it can be called administrative-economic regional division. If administrative division is not consistent with economic regional division, then it can be called non-administrative - economic region, i.e.