University of Nevada, Reno INVESTIGATION of the ROLE for TERMINAL SCHWANN CELLS in NEUROMUSCULAR JUNCTION DEVELOPMENT and DISEAS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mir-338-3P Functions As a Tumor Suppressor in Gastric Cancer by Targeting PTP1B
Sun et al. Cell Death and Disease DOI 10.1038/s41419-018-0611-0 Cell Death & Disease ARTICLE Open Access miR-338-3p functions as a tumor suppressor in gastric cancer by targeting PTP1B Feng Sun1, Mengchao Yu2,JingYu2, Zhijian Liu1,XinyanZhou2,YanqingLiu2, Xiaolong Ge3,HaidongGao2, Mei Li4, Xiaohong Jiang2,SongLiu1,XiChen2 and Wenxian Guan 1 Abstract Gastric cancer (GC) is one of the most common malignant tumors and peritoneal metastasis is the primary cause for advanced GC’s mortality. Protein-tyrosine phosphatase 1B (PTP1B) functions as an oncogene and involves in carcinogenesis and cancer dissemination. However, the function and regulation of PTP1B in GC remain poorly understood. In this study, we found that PTP1B was upregulated in GC tissues and overexpression of PTP1B in vitro promoted cell migration and prevented apoptosis. Then, we predicted that PTP1B was a target of miR-338-3p and we revealed an inverse correlation between miR-338-3p levels and PTP1B protein levels in GC tissues. Next, we verified that PTP1B was inhibited by miR-338-3p via direct targeting to its 3′-untranslated regions. Moreover, overexpression of miR-338-3p in vitro attenuated GC cell migration and promoted apoptosis, and these effects could be partially reversed by reintroduction of PTP1B. Finally, we established an orthotopic xenograft model and a peritoneal dissemination model of GC to demonstrate that miR-338-3p restrained tumor growth and dissemination in vivo by targeting PTP1B. Taken together, our results highlight that PTP1B is an oncogene and is negatively regulated by miR- 1234567890():,; 1234567890():,; 338-3p in GC, which may provide new insights into novel molecular therapeutic targets for GC. -

EP3 Prostate Adenocarcinoma Metastasis to the Bilateral Ureters
Autopsy, Forensic, Grossing 004 Id: EP3 Prostate Adenocarcinoma Metastasis to the Bilateral Ureters: An Unusual Pattern Suvra Roy, MD, L. Maximilian Buja, MD, University of Texas Health Science Center at Houston We report an autopsy of a patient with prostate cancer who had hydronephrosis and sepsis due to obstruction from Downloaded from https://academic.oup.com/ajcp/article/144/suppl_2/A004/1772163 by guest on 23 September 2021 bilateral ureteral metastasis of prostate adenocarcinoma. He was an 82-year-old man who presented to the emergency department with weakness and shortness of breath. Fifteen years earlier, he had been diagnosed with prostate cancer , underwent chemotherapy, and was in remission for 10 years. Eighteen months ago, he developed a recurrence and began chemotherapy again but, because of his worsening renal condition, stopped the chemotherapy about 4 months ago. On admission, he was found to have chronic kidney disease, stage 5, and sepsis. Abdominal CT was negative for genitourinary mass. His condition deteriorated rapidly and he developed bradycardia and then pulseless electrical activity (PEA). He went into cardiac arrest for 30 minutes without return of pulse and remained in PEA. His poor prognosis was explained to his family and, per the family’s wishes, resuscitation was stopped, and the patient died. Autopsy revealed bilateral dilated renal pelvis, trabeculated urinary bladder and enlarged prostate. No gross evidence of metastasis was identified in lymph nodes or bone. However, the openings of the ureters revealed papillary masses involving the distal ureters bilaterally, but not involving the bladder. Microscopic examination of the masses revealed atypical tumor cells with highly pleomorphic features. -

The Role of Z-Disc Proteins in Myopathy and Cardiomyopathy
International Journal of Molecular Sciences Review The Role of Z-disc Proteins in Myopathy and Cardiomyopathy Kirsty Wadmore 1,†, Amar J. Azad 1,† and Katja Gehmlich 1,2,* 1 Institute of Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, UK; [email protected] (K.W.); [email protected] (A.J.A.) 2 Division of Cardiovascular Medicine, Radcliffe Department of Medicine and British Heart Foundation Centre of Research Excellence Oxford, University of Oxford, Oxford OX3 9DU, UK * Correspondence: [email protected]; Tel.: +44-121-414-8259 † These authors contributed equally. Abstract: The Z-disc acts as a protein-rich structure to tether thin filament in the contractile units, the sarcomeres, of striated muscle cells. Proteins found in the Z-disc are integral for maintaining the architecture of the sarcomere. They also enable it to function as a (bio-mechanical) signalling hub. Numerous proteins interact in the Z-disc to facilitate force transduction and intracellular signalling in both cardiac and skeletal muscle. This review will focus on six key Z-disc proteins: α-actinin 2, filamin C, myopalladin, myotilin, telethonin and Z-disc alternatively spliced PDZ-motif (ZASP), which have all been linked to myopathies and cardiomyopathies. We will summarise pathogenic variants identified in the six genes coding for these proteins and look at their involvement in myopathy and cardiomyopathy. Listing the Minor Allele Frequency (MAF) of these variants in the Genome Aggregation Database (GnomAD) version 3.1 will help to critically re-evaluate pathogenicity based on variant frequency in normal population cohorts. -

Germ Cell Origin of Testicular Carcinoid Tumors Phillip H
Imaging, Diagnosis, Prognosis Germ Cell Origin of Testicular Carcinoid Tumors Phillip H. Abbosh,1Shaobo Zhang,1Gregory T.MacLennan,3 Rodolfo Montironi,4 Antonio Lopez-Beltran,5 Joseph P. Rank,6 LeeAnn Baldridge,1and Liang Cheng1, 2 Abstract Purpose: Carcinoids are neuroendocrine tumors and most frequently occur within tissues derived from the embryonic gut.These tumors can occur in any organ site but are rare in the testis. The cell type giving rise to testicular carcinoid is unknown.We hypothesized that testicular carci- noid may have a germ cell origin. Experimental Design: We describe our analysis of protein and genetic markers of germ cell neoplasia, using immunohistochemistry and fluorescence in situ hybridization, in four testicular carcinoid tumors. Results: All four cases of testicular carcinoid tumor arose in a background of mature teratoma. Isochromosome 12p was identified in carcinoid tumor cells in all four samples. 12p overrepresen- tation was also observed in three cases. Isochromosome 12p and 12p overrepresentation were present in cells of coexisting mature teratoma in three cases. Carcinoid tumors showed strong immunoreactivity for synaptophysin and chromogranin, but no immunoreactivity for OCT4, CD30, c-kit,TTF-1, and CDX2. Membranous and cytoplasmic staining for h-catenin was detected in three cases. Conclusion: Our findings suggest that testicular carcinoid represents a phenotypic expression of testicular teratoma and is of germ cell origin. Testicular carcinoid tumor is rare. It was originally reported in Materials and Methods 1954 by Simon (1) who described it as part of a cystic teratoma, and additional cases have been subsequently reported. All Patients. We analyzed four cases of testicular carcinoid tumor. -
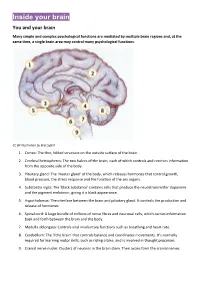
Inside Your Brain You and Your Brain
Inside your brain You and your brain Many simple and complex psychological functions are mediated by multiple brain regions and, at the same time, a single brain area may control many psychological functions. CC BY Illustration by Bret Syfert 1. Cortex: The thin, folded structure on the outside surface of the brain. 2. Cerebral hemispheres: The two halves of the brain, each of which controls and receives information from the opposite side of the body. 3. Pituitary gland: The ‘master gland’ of the body, which releases hormones that control growth, blood pressure, the stress response and the function of the sex organs. 4. Substantia nigra: The ‘black substance’ contains cells that produce the neurotransmitter dopamine and the pigment melatonin, giving it a black appearance. 5. Hypothalamus: The interface between the brain and pituitary gland. It controls the production and release of hormones. 6. Spinal cord: A large bundle of millions of nerve fibres and neuronal cells, which carries information back and forth between the brain and the body. 7. Medulla oblongata: Controls vital involuntary functions such as breathing and heart rate. 8. Cerebellum: The ‘little brain’ that controls balance and coordinates movements. It’s normally required for learning motor skills, such as riding a bike, and is involved in thought processes. 9. Cranial nerve nuclei: Clusters of neurons in the brain stem. Their axons form the cranial nerves. Your brain underpins who you are. It stores your knowledge and memories, gives you the capacity for thought and emotion, and enables you to control your body. The brain is just one part of the nervous system. -

Neuroendocrine Differentiation of Prostatic Adenocarcinoma
J Lab Med 2019; 43(2): 123–126 Laboratory Case Report Cátia Iracema Morais*, João Lobo, João P. Barreto, Cláudia Lobo and Nuno D. Gonçalves Neuroendocrine differentiation of prostatic adenocarcinoma – an important cause for castration-resistant disease recurrence https://doi.org/10.1515/labmed-2018-0190 awareness of this entity is crucial due to its underdiagno- Received December 3, 2018; accepted December 12, 2018; previously sis and adverse prognosis. published online February 15, 2019 Keywords: carcinoma; castration-resistant (D064129); cell Abstract transformation; neoplastic (D002471); neuroendocrine (D018278); prostate (D011467); prostatic neoplasms. Background: Neuroendocrine differentiation of prostatic carcinoma is a rare entity associated with metastatic castration-resistant disease. Among useful biomarkers of neuroendocrine differentiation, chromogranin A, sero- Introduction tonin, synaptophysin and neuron-specific enolase stand out, while total prostate-specific antigen (PSA) levels are Neuroendocrine prostatic carcinoma is a rare and often low or undetectable. underdiagnosed histologic subtype. Despite the low Case presentation: We report a case of prostatic adenocar- incidence rate of primary neuroendocrine prostatic cinoma recurrence after a 6-year disease-free follow-up, in carcinoma (which represents under 1% of all prostate which increased serum chromogranin A levels and unde- cancers at diagnosis), 30–40% of patients who develop tectable total PSA provided a prompt indication of neu- metastasized castration-resistant -

Transiently Structured Head Domains Control Intermediate Filament Assembly
Transiently structured head domains control intermediate filament assembly Xiaoming Zhoua, Yi Lina,1, Masato Katoa,b,c, Eiichiro Morid, Glen Liszczaka, Lillian Sutherlanda, Vasiliy O. Sysoeva, Dylan T. Murraye, Robert Tyckoc, and Steven L. McKnighta,2 aDepartment of Biochemistry, University of Texas Southwestern Medical Center, Dallas, TX 75390; bInstitute for Quantum Life Science, National Institutes for Quantum and Radiological Science and Technology, 263-8555 Chiba, Japan; cLaboratory of Chemical Physics, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD 20892-0520; dDepartment of Future Basic Medicine, Nara Medical University, 840 Shijo-cho, Kashihara, Nara, Japan; and eDepartment of Chemistry, University of California, Davis, CA 95616 Contributed by Steven L. McKnight, January 2, 2021 (sent for review October 30, 2020; reviewed by Lynette Cegelski, Tatyana Polenova, and Natasha Snider) Low complexity (LC) head domains 92 and 108 residues in length are, IF head domains might facilitate filament assembly in a manner respectively, required for assembly of neurofilament light (NFL) and analogous to LC domain function by RNA-binding proteins in the desmin intermediate filaments (IFs). As studied in isolation, these IF assembly of RNA granules. head domains interconvert between states of conformational disor- IFs are defined by centrally located α-helical segments 300 to der and labile, β-strand–enriched polymers. Solid-state NMR (ss-NMR) 350 residues in length. These central, α-helical segments are spectroscopic studies of NFL and desmin head domain polymers re- flanked on either end by head and tail domains thought to be veal spectral patterns consistent with structural order. -

Limited Diagnostic Utility of Chromogranin a Measurements in Workup of Neuroendocrine Tumors
diagnostics Article Limited Diagnostic Utility of Chromogranin A Measurements in Workup of Neuroendocrine Tumors Jonas Baekdal 1,2,*, Jesper Krogh 1,2, Marianne Klose 1,2, Pernille Holmager 1,2, Seppo W. Langer 1,3, Peter Oturai 1,4,5, Andreas Kjaer 1,4,5 , Birgitte Federspiel 1,6, Linda Hilsted 1,7, Jens F. Rehfeld 1,7, Ulrich Knigge 1,2,8 and Mikkel Andreassen 1,2 1 ENETS Neuroendocrine Tumor Centre of Excellence, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark; [email protected] (J.K.); [email protected] (M.K.); [email protected] (P.H.); [email protected] (S.W.L.); [email protected] (P.O.); [email protected] (A.K.); [email protected] (B.F.); [email protected] (L.H.); [email protected] (J.F.R.); [email protected] (U.K.); [email protected] (M.A.) 2 Department of Endocrinology, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark 3 Department of Oncology, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark 4 Department of Clinical Physiology, Nuclear Medicine & PET and Cluster for Molecular Imaging, Copenhagen University Hospital, 2100 Copenhagen, Denmark 5 Department of Biomedical Sciences, Rigshospitalet and University of Copenhagen, 2100 Copenhagen, Denmark 6 Department of Pathology, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark 7 Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark 8 Department of Surgery and Transplantation, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark * Correspondence: [email protected]; Tel.: +45-6013-4687 Received: 11 October 2020; Accepted: 28 October 2020; Published: 29 October 2020 Abstract: Background: Plasma chromogranin A (CgA) is related to tumor burden and recommended in the follow-up of patients diagnosed with neuroendocrine tumors (NETs). -

Ponatinib Shows Potent Antitumor Activity in Small Cell Carcinoma of the Ovary Hypercalcemic Type (SCCOHT) Through Multikinase Inhibition Jessica D
Published OnlineFirst February 9, 2018; DOI: 10.1158/1078-0432.CCR-17-1928 Cancer Therapy: Preclinical Clinical Cancer Research Ponatinib Shows Potent Antitumor Activity in Small Cell Carcinoma of the Ovary Hypercalcemic Type (SCCOHT) through Multikinase Inhibition Jessica D. Lang1,William P.D. Hendricks1, Krystal A. Orlando2, Hongwei Yin1, Jeffrey Kiefer1, Pilar Ramos1, Ritin Sharma3, Patrick Pirrotte3, Elizabeth A. Raupach1,3, Chris Sereduk1, Nanyun Tang1, Winnie S. Liang1, Megan Washington1, Salvatore J. Facista1, Victoria L. Zismann1, Emily M. Cousins4, Michael B. Major4, Yemin Wang5, Anthony N. Karnezis5, Aleksandar Sekulic1,6, Ralf Hass7, Barbara C. Vanderhyden8, Praveen Nair9, Bernard E. Weissman2, David G. Huntsman5,10, and Jeffrey M. Trent1 Abstract Purpose: Small cell carcinoma of the ovary, hypercalcemic type three SWI/SNF wild-type ovarian cancer cell lines. We further (SCCOHT) is a rare, aggressive ovarian cancer in young women identified ponatinib as the most effective clinically approved that is universally driven by loss of the SWI/SNF ATPase subunits RTK inhibitor. Reexpression of SMARCA4 was shown to confer SMARCA4 and SMARCA2. A great need exists for effective targeted a 1.7-fold increase in resistance to ponatinib. Subsequent therapies for SCCOHT. proteomic assessment of ponatinib target modulation in Experimental Design: To identify underlying therapeutic vul- SCCOHT cell models confirmed inhibition of nine known nerabilities in SCCOHT, we conducted high-throughput siRNA ponatinib target kinases alongside 77 noncanonical ponatinib and drug screens. Complementary proteomics approaches pro- targets in SCCOHT. Finally, ponatinib delayed tumor dou- filed kinases inhibited by ponatinib. Ponatinib was tested for bling time 4-fold in SCCOHT-1 xenografts while reducing efficacy in two patient-derived xenograft (PDX) models and one final tumor volumes in SCCOHT PDX models by 58.6% and cell-line xenograft model of SCCOHT. -
Hyperactive and Impulsive Behaviors of LMTK1 Knockout Mice
www.nature.com/scientificreports OPEN Hyperactive and impulsive behaviors of LMTK1 knockout mice Miyuki Takahashi1,7, Arika Sugiyama1, Ran Wei1, Shizuka Kobayashi2, Kimiko Fukuda3, Hironori Nishino1, Roka Takahashi1, Koji Tsutsumi1,8, Ichiro Kita4, Kanae Ando1, Toshiya Manabe2, Hiroyuki Kamiguchi5, Mineko Tomomura6 & Shin‑ichi Hisanaga1* Lemur tail kinase 1 (LMTK1), previously called Apoptosis‑Associated Tyrosine Kinase (AATYK), remains an uncharacterized Ser/Thr protein kinase that is predominantly expressed in the brain. It is recently reported that LMTK1A, an isoform of LMTK1, binds to recycling endosomes through its palmitoylation and regulates endosomal trafcking by suppressing the activity of Rab11 small GTPase. In neurons, knockdown or knockout of LMTK1 results in longer axons, greater branching of dendrites and increased number of spines, suggesting that LMTK1 plays a role in neuronal circuit formation. However, its in vivo function remained to be investigated. Here, we examined the brain structures and behaviors of LMTK1 knockout (KO) mice. LMTK1 was expressed in most neurons throughout the brain. The overall brain structure appeared to be normal in LMTK1 KO mice, but the numbers of synapses were increased. LMTK1 KO mice had a slight impairment in memory formation and exhibited distinct psychiatric behaviors such as hyperactivity, impulsiveness and high motor coordination without social interaction defcits. Some of these abnormal behaviors represent core features of attention defcit hyperactive disorder (ADHD), suggesting the possible involvement of LMTK1 in the pathogenesis of ADHD. Abbreviations AATYK1 Apoptosis-Associated Tyrosine Kinase ADHD Attention defcit hyperactive disorder Cx Cerebral cortex Cb Cerebellum CS Conditioned stimulus ISH In situ hybridization KO Knockout LMTK Lemur tail kinase PPF Paired-pulse facilitation US Unconditioned stimulus WT Wildtype Neuronal circuit wiring is the structural and functional basis of higher brain activities such as recognition and behaviors. -

1714 Gene Comprehensive Cancer Panel Enriched for Clinically Actionable Genes with Additional Biologically Relevant Genes 400-500X Average Coverage on Tumor
xO GENE PANEL 1714 gene comprehensive cancer panel enriched for clinically actionable genes with additional biologically relevant genes 400-500x average coverage on tumor Genes A-C Genes D-F Genes G-I Genes J-L AATK ATAD2B BTG1 CDH7 CREM DACH1 EPHA1 FES G6PC3 HGF IL18RAP JADE1 LMO1 ABCA1 ATF1 BTG2 CDK1 CRHR1 DACH2 EPHA2 FEV G6PD HIF1A IL1R1 JAK1 LMO2 ABCB1 ATM BTG3 CDK10 CRK DAXX EPHA3 FGF1 GAB1 HIF1AN IL1R2 JAK2 LMO7 ABCB11 ATR BTK CDK11A CRKL DBH EPHA4 FGF10 GAB2 HIST1H1E IL1RAP JAK3 LMTK2 ABCB4 ATRX BTRC CDK11B CRLF2 DCC EPHA5 FGF11 GABPA HIST1H3B IL20RA JARID2 LMTK3 ABCC1 AURKA BUB1 CDK12 CRTC1 DCUN1D1 EPHA6 FGF12 GALNT12 HIST1H4E IL20RB JAZF1 LPHN2 ABCC2 AURKB BUB1B CDK13 CRTC2 DCUN1D2 EPHA7 FGF13 GATA1 HLA-A IL21R JMJD1C LPHN3 ABCG1 AURKC BUB3 CDK14 CRTC3 DDB2 EPHA8 FGF14 GATA2 HLA-B IL22RA1 JMJD4 LPP ABCG2 AXIN1 C11orf30 CDK15 CSF1 DDIT3 EPHB1 FGF16 GATA3 HLF IL22RA2 JMJD6 LRP1B ABI1 AXIN2 CACNA1C CDK16 CSF1R DDR1 EPHB2 FGF17 GATA5 HLTF IL23R JMJD7 LRP5 ABL1 AXL CACNA1S CDK17 CSF2RA DDR2 EPHB3 FGF18 GATA6 HMGA1 IL2RA JMJD8 LRP6 ABL2 B2M CACNB2 CDK18 CSF2RB DDX3X EPHB4 FGF19 GDNF HMGA2 IL2RB JUN LRRK2 ACE BABAM1 CADM2 CDK19 CSF3R DDX5 EPHB6 FGF2 GFI1 HMGCR IL2RG JUNB LSM1 ACSL6 BACH1 CALR CDK2 CSK DDX6 EPOR FGF20 GFI1B HNF1A IL3 JUND LTK ACTA2 BACH2 CAMTA1 CDK20 CSNK1D DEK ERBB2 FGF21 GFRA4 HNF1B IL3RA JUP LYL1 ACTC1 BAG4 CAPRIN2 CDK3 CSNK1E DHFR ERBB3 FGF22 GGCX HNRNPA3 IL4R KAT2A LYN ACVR1 BAI3 CARD10 CDK4 CTCF DHH ERBB4 FGF23 GHR HOXA10 IL5RA KAT2B LZTR1 ACVR1B BAP1 CARD11 CDK5 CTCFL DIAPH1 ERCC1 FGF3 GID4 HOXA11 IL6R KAT5 ACVR2A -

Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems
cells Review Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems Naoki Abe 1, Tasuku Nishihara 1,*, Toshihiro Yorozuya 1 and Junya Tanaka 2 1 Department of Anesthesia and Perioperative Medicine, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; [email protected] (N.A.); [email protected] (T.Y.) 2 Department of Molecular and cellular Physiology, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-89-960-5383; Fax: +81-89-960-5386 Received: 4 August 2020; Accepted: 17 September 2020; Published: 21 September 2020 Abstract: Microglia, the immunocompetent cells in the central nervous system (CNS), have long been studied as pathologically deteriorating players in various CNS diseases. However, microglia exert ameliorating neuroprotective effects, which prompted us to reconsider their roles in CNS and peripheral nervous system (PNS) pathophysiology. Moreover, recent findings showed that microglia play critical roles even in the healthy CNS. The microglial functions that normally contribute to the maintenance of homeostasis in the CNS are modified by other cells, such as astrocytes and infiltrated myeloid cells; thus, the microglial actions on neurons are extremely complex. For a deeper understanding of the pathophysiology of various diseases, including those of the PNS, it is important to understand microglial functioning. In this review, we discuss both the favorable and unfavorable roles of microglia in neuronal survival in various CNS and PNS disorders. We also discuss the roles of blood-borne macrophages in the pathogenesis of CNS and PNS injuries because they cooperatively modify the pathological processes of resident microglia.