The Role of the Propeptide and Its Residues in Activation and Secretion of Elastase, an M4 Metalloprotease Secreted by Pseudomonas Aeruginosa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A-Ailable At
International Journal of Current Advanced Research ISSN: O: 2319-6475, ISSN: P: 2319 – 6505, Impact Factor: SJIF: 5.438 Available Online at www.journalijcar.org Volume 6; Issue 2; February 2017; Page No. 2131-2138 Research Article A COMPARATIVE ANALYSIS OF THERMOPHILIC AND PSYCHROPHILIC METALLOPEPTIDASES: INVOLVEMENT OF BIOINFORMATIC APPROACH Anuprabha1., AbhigyanNath2 and Radha Chaube3* 1,2 3Bioinformatic Division, MahilaMahavidyalya, Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India ARTICLEDepartment INFO of Zoology, Institute of Science,ABSTRACT Banaras Hindu University, Varanasi-221005, Uttar Pradesh, India The thermolysin family of enzymes is classified as the M4 family of metallopeptidases. M4 Article History: family comprises numerous zinc-dependent metallopeptidases that hydrolyze peptide Received 9th November, 2016 bonds. Zinc containing peptidases are widely distributed in nature and have important roles Received in revised form 12thDecember, 2016 in many physiological processes. M4 family comprises numerous zinc-dependent Accepted 5th January, 2017 metallopeptidases that hydrolyse peptide bonds. Metallopeptidases were studied for the Published online 28th February, 2017 reason of their great relevance to biology, medicine and biotechnology. In the present study, detailed comparative analyses of both thermophilic and psychrophilic metallopeptidases were performed. The comparative analysis of both thermophilic and Key words: psychrophilic metallopeptidases was performed by taking sequence parameters such as Thermophilic, Psychrophilic, amino acid composition, amino acid property group composition, physico-chemical Metallopeptidases, amino acid residues, properties, secondary structure content. From comparison between the two groups, it was analysis found that Gln, Asn, Ser, Thr, and His are significantly lower in thermophilic metallopeptidase. Positively charged residues (Lys, Arg and Glu) are more significant in thermophilic metallopeptidases than in psychrophilic metallopeptidases. -

(12) United States Patent (10) Patent No.: US 6,395,889 B1 Robison (45) Date of Patent: May 28, 2002
USOO6395889B1 (12) United States Patent (10) Patent No.: US 6,395,889 B1 Robison (45) Date of Patent: May 28, 2002 (54) NUCLEIC ACID MOLECULES ENCODING WO WO-98/56804 A1 * 12/1998 ........... CO7H/21/02 HUMAN PROTEASE HOMOLOGS WO WO-99/0785.0 A1 * 2/1999 ... C12N/15/12 WO WO-99/37660 A1 * 7/1999 ........... CO7H/21/04 (75) Inventor: fish E. Robison, Wilmington, MA OTHER PUBLICATIONS Vazquez, F., et al., 1999, “METH-1, a human ortholog of (73) Assignee: Millennium Pharmaceuticals, Inc., ADAMTS-1, and METH-2 are members of a new family of Cambridge, MA (US) proteins with angio-inhibitory activity', The Journal of c: - 0 Biological Chemistry, vol. 274, No. 33, pp. 23349–23357.* (*) Notice: Subject to any disclaimer, the term of this Descriptors of Protease Classes in Prosite and Pfam Data patent is extended or adjusted under 35 bases. U.S.C. 154(b) by 0 days. * cited by examiner (21) Appl. No.: 09/392, 184 Primary Examiner Ponnathapu Achutamurthy (22) Filed: Sep. 9, 1999 ASSistant Examiner William W. Moore (51) Int. Cl." C12N 15/57; C12N 15/12; (74) Attorney, Agent, or Firm-Alston & Bird LLP C12N 9/64; C12N 15/79 (57) ABSTRACT (52) U.S. Cl. .................... 536/23.2; 536/23.5; 435/69.1; 435/252.3; 435/320.1 The invention relates to polynucleotides encoding newly (58) Field of Search ............................... 536,232,235. identified protease homologs. The invention also relates to 435/6, 226, 69.1, 252.3 the proteases. The invention further relates to methods using s s s/ - - -us the protease polypeptides and polynucleotides as a target for (56) References Cited diagnosis and treatment in protease-mediated disorders. -

The Extracellular Proteases Produced by Vibrio Parahaemolyticus
World Journal of Microbiology and Biotechnology (2018) 34:68 https://doi.org/10.1007/s11274-018-2453-4 REVIEW The extracellular proteases produced by Vibrio parahaemolyticus George Osei‑Adjei1 · Xinxiang Huang1 · Yiquan Zhang1 Received: 20 February 2018 / Accepted: 8 May 2018 © Springer Science+Business Media B.V., part of Springer Nature 2018 Abstract Vibrio parahaemolyticus, a Gram-negative bacterium, inhabits marine and estuarine environments and it is a major pathogen responsible globally for most cases of seafood-associated gastroenteritis in humans and acute hepatopancreatic necrosis syn- drome in shrimps. There has been a dramatic worldwide increase in V. parahaemolyticus infections over the last two decades. The pathogenicity of V. parahaemolyticus has been linked to the expression of different kinds of virulence factors including extracellular proteases, such as metalloproteases and serine proteases. V. parahaemolyticus expresses the metalloproteases; PrtV, VppC, VPM and the serine proteases; VPP1/Protease A, VpSP37, PrtA. Extracellular proteases have been identified as potential virulence factors which directly digest many kinds of host proteins or indirectly are involved in the processing of other toxic protein factors. This review summarizes findings on the metalloproteases and serine proteases produced by V. parahaemolyticus and their roles in infections. Identifying the role of V. parahaemolyticus virulence-associated extracellular proteases deepens our understanding of diseases caused by this bacterium. Keywords Extracellular protease · Metalloprotease · Serine protease · Vibrio parahaemolyticus · Virulence Introduction incubation period of V. parahaemolyticus is usually 12–24 h post-infection with infection usually occurring in the hot The Gram-negative bacterium, Vibrio parahaemolyticus, summer months because the bacteria is found free swim- lives in marine and estuarine environments and it is a major ming in coastal water or attached to fish and shellfish. -

Serine Proteases with Altered Sensitivity to Activity-Modulating
(19) & (11) EP 2 045 321 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.04.2009 Bulletin 2009/15 C12N 9/00 (2006.01) C12N 15/00 (2006.01) C12Q 1/37 (2006.01) (21) Application number: 09150549.5 (22) Date of filing: 26.05.2006 (84) Designated Contracting States: • Haupts, Ulrich AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 51519 Odenthal (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • Coco, Wayne SK TR 50737 Köln (DE) •Tebbe, Jan (30) Priority: 27.05.2005 EP 05104543 50733 Köln (DE) • Votsmeier, Christian (62) Document number(s) of the earlier application(s) in 50259 Pulheim (DE) accordance with Art. 76 EPC: • Scheidig, Andreas 06763303.2 / 1 883 696 50823 Köln (DE) (71) Applicant: Direvo Biotech AG (74) Representative: von Kreisler Selting Werner 50829 Köln (DE) Patentanwälte P.O. Box 10 22 41 (72) Inventors: 50462 Köln (DE) • Koltermann, André 82057 Icking (DE) Remarks: • Kettling, Ulrich This application was filed on 14-01-2009 as a 81477 München (DE) divisional application to the application mentioned under INID code 62. (54) Serine proteases with altered sensitivity to activity-modulating substances (57) The present invention provides variants of ser- screening of the library in the presence of one or several ine proteases of the S1 class with altered sensitivity to activity-modulating substances, selection of variants with one or more activity-modulating substances. A method altered sensitivity to one or several activity-modulating for the generation of such proteases is disclosed, com- substances and isolation of those polynucleotide se- prising the provision of a protease library encoding poly- quences that encode for the selected variants. -

The Positive Side of Proteolysis in Alzheimer's Disease
Hindawi Publishing Corporation Biochemistry Research International Volume 2011, Article ID 721463, 13 pages doi:10.1155/2011/721463 Review Article Zinc Metalloproteinases and Amyloid Beta-Peptide Metabolism: The Positive Side of Proteolysis in Alzheimer’s Disease Mallory Gough, Catherine Parr-Sturgess, and Edward Parkin Division of Biomedical and Life Sciences, School of Health and Medicine, Lancaster University, Lancaster LA1 4YQ, UK Correspondence should be addressed to Edward Parkin, [email protected] Received 17 August 2010; Accepted 7 September 2010 Academic Editor: Simon J. Morley Copyright © 2011 Mallory Gough et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Alzheimer’s disease is a neurodegenerative condition characterized by an accumulation of toxic amyloid beta- (Aβ-)peptides in the brain causing progressive neuronal death. Aβ-peptides are produced by aspartyl proteinase-mediated cleavage of the larger amyloid precursor protein (APP). In contrast to this detrimental “amyloidogenic” form of proteolysis, a range of zinc metalloproteinases can process APP via an alternative “nonamyloidogenic” pathway in which the protein is cleaved within its Aβ region thereby precluding the formation of intact Aβ-peptides. In addition, other members of the zinc metalloproteinase family can degrade preformed Aβ-peptides. As such, the zinc metalloproteinases, collectively, are key to downregulating Aβ generation and enhancing its degradation. It is the role of zinc metalloproteinases in this “positive side of proteolysis in Alzheimer’s disease” that is discussed in the current paper. 1. Introduction of 38–43 amino acid peptides called amyloid beta (Aβ)- peptides. -

Secretion of Matrix Metalloproteinases and Their
[CANCER RESEARCH 53, 4493-4498, October 1, 1993] Secretion of Matrix Metalloproteinases and Their Inhibitors (Tissue Inhibitor of Metalloproteinases) by Human Prostate in Explant Cultures: Reduced Tissue Inhibitor of Metailoproteinase Secretion by Malignant Tissues 1 Balakrishna L. Lokeshwar, 2 Marie G. Seizer, Norman L. Block, and Zeenat Gunja-Smith Departments of Urology [B. L. L., M. G. S., N. L. B.] and Medicine [Z. G-S.], University of Miami School of Medicine, Miami, Florida 33101 ABSTRACT V, and IX, as well as the noncollagenous components of the extracel- lular matrix, including laminin and fibronectin (6). In addition, Unregulated secretion of matrix metalloproteinases (MMPs) or their MMP-3 is also known to activate procollagenases (7). Increased levels endogenous protein inhibitors (tissue inhibitor of metalloproteinases, of these proteinases have been implicated with the invasive potential TIMPs) has been implicated in tumor invasion and metastasis. Species of MMPs and TIMPs secreted by epithelial cultures of normal, benign, and of tumors (8-10). malignant prostate were identified and their levels were compared. Frag- Matrixins secreted by normal cells are proenzymes (zymogens) and ments of fresh tissue were cultured in a serum-free medium that supported are inactive (11). This is due to a highly conserved sequence of nine the outgrowth of prostatic epithelial cells. Biochemical analysis of the amino acid residues in the propeptide region containing a solitary conditioned media by gelatin zymography and enzyme assays showed that cysteine residue bound to the metal ion, Zn 2+, at the active center both normal and neoplastic tissues secreted latent and active forms of both (12). -

Zinc Metalloproteinases and Amyloid Beta-Peptide Metabolism: the Positive Side of Proteolysis in Alzheimer’S Disease
Hindawi Publishing Corporation Biochemistry Research International Volume 2011, Article ID 721463, 13 pages doi:10.1155/2011/721463 Review Article Zinc Metalloproteinases and Amyloid Beta-Peptide Metabolism: The Positive Side of Proteolysis in Alzheimer’s Disease Mallory Gough, Catherine Parr-Sturgess, and Edward Parkin Division of Biomedical and Life Sciences, School of Health and Medicine, Lancaster University, Lancaster LA1 4YQ, UK Correspondence should be addressed to Edward Parkin, [email protected] Received 17 August 2010; Accepted 7 September 2010 Academic Editor: Simon J. Morley Copyright © 2011 Mallory Gough et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Alzheimer’s disease is a neurodegenerative condition characterized by an accumulation of toxic amyloid beta- (Aβ-)peptides in the brain causing progressive neuronal death. Aβ-peptides are produced by aspartyl proteinase-mediated cleavage of the larger amyloid precursor protein (APP). In contrast to this detrimental “amyloidogenic” form of proteolysis, a range of zinc metalloproteinases can process APP via an alternative “nonamyloidogenic” pathway in which the protein is cleaved within its Aβ region thereby precluding the formation of intact Aβ-peptides. In addition, other members of the zinc metalloproteinase family can degrade preformed Aβ-peptides. As such, the zinc metalloproteinases, collectively, are key to downregulating Aβ generation and enhancing its degradation. It is the role of zinc metalloproteinases in this “positive side of proteolysis in Alzheimer’s disease” that is discussed in the current paper. 1. Introduction of 38–43 amino acid peptides called amyloid beta (Aβ)- peptides. -

Polymorphisms of the Matrix Metalloproteinase Genes
www.nature.com/scientificreports OPEN Polymorphisms of the matrix metalloproteinase genes are associated with essential hypertension in a Caucasian population of Central Russia Maria Moskalenko1, Irina Ponomarenko1, Evgeny Reshetnikov1*, Volodymyr Dvornyk2 & Mikhail Churnosov1 This study aimed to determine possible association of eight polymorphisms of seven MMP genes with essential hypertension (EH) in a Caucasian population of Central Russia. Eight SNPs of the MMP1, MMP2, MMP3, MMP7, MMP8, MMP9, and MMP12 genes and their gene–gene (epistatic) interactions were analyzed for association with EH in a cohort of 939 patients and 466 controls using logistic regression and assuming additive, recessive, and dominant genetic models. The functional signifcance of the polymorphisms associated with EH and 114 variants linked to them (r2 ≥ 0.8) was analyzed in silico. Allele G of rs11568818 MMP7 was associated with EH according to all three genetic models (OR = 0.58–0.70, pperm = 0.01–0.03). The above eight SNPs were associated with the disorder within 12 most signifcant epistatic models (OR = 1.49–1.93, pperm < 0.02). Loci rs1320632 MMP8 and rs11568818 MMP7 contributed to the largest number of the models (12 and 10, respectively). The EH-associated loci and 114 SNPs linked to them had non-synonymous, regulatory, and eQTL signifcance for 15 genes, which contributed to the pathways related to metalloendopeptidase activity, collagen degradation, and extracellular matrix disassembly. In summary, eight studied SNPs of MMPs genes were associated with EH in the Caucasian population of Central Russia. Cardiovascular diseases are a global problem of modern healthcare and the second most common cause of total mortality1,2. -
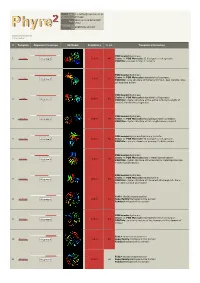
Phyre 2 Results for P22894
Email [email protected] Description P22894 Wed Jun 6 09:54:50 BST Date 2012 Unique Job eba6f50a2e10c91f ID Detailed template information # Template Alignment Coverage 3D Model Confidence % i.d. Template Information PDB header:hydrolase 1 c1gxdA_ Alignment 100.0 46 Chain: A: PDB Molecule:72 kda type iv collagenase; PDBTitle: prommp-2/timp-2 complex PDB header:hydrolase Chain: A: PDB Molecule:interstitial collagenase; 2 c1su3A_ 100.0 61 Alignment PDBTitle: x-ray structure of human prommp-1: new insights into2 collagenase action PDB header:hydrolase Chain: B: PDB Molecule:interstitial collagenase; 3 c2cltB_ 100.0 61 Alignment PDBTitle: crystal structure of the active form (full-length) of human2 fibroblast collagenase. PDB header:hydrolase 4 c3ba0A_ Alignment 100.0 49 Chain: A: PDB Molecule:macrophage metalloelastase; PDBTitle: crystal structure of full-length human mmp-12 PDB header:hydrolase/hydrolase inhibitor 5 c1eakA_ Alignment 100.0 52 Chain: A: PDB Molecule:72 kda type iv collagenase; PDBTitle: catalytic domain of prommp-2 e404q mutant PDB header:hydrolase Chain: A: PDB Molecule:matrix metalloproteinase-9; 6 c1l6jA_ 100.0 56 Alignment PDBTitle: crystal structure of human matrix metalloproteinase mmp92 (gelatinase b). PDB header:hydrolase Chain: A: PDB Molecule:stromelysin-1; 7 c1slmA_ 100.0 54 Alignment PDBTitle: crystal structure of fibroblast stromelysin-1: the c- truncated human2 proenzyme Fold:4-bladed beta-propeller 8 d1fbla1 Alignment 100.0 57 Superfamily:Hemopexin-like domain Family:Hemopexin-like domain PDB header:hydrolase -

Modification of the Transcriptomic Response to Renal Ischemia
Kidney International, Vol. 64 (2003), pp. 480–492 CELL BIOLOGY–IMMUNOLOGY–PATHOLOGY Modification of the transcriptomic response to renal ischemia/ reperfusion injury by lipoxin analog NIAMH E. KIERAN,1 PETER P. DORAN,1 SUSAN B. CONNOLLY,MARIE-CLAIRE GREENAN, DEBRA F. HIGGINS,MARTIN LEONARD,CATHERINE GODSON,CORMAC T. TAYLOR, ANNA HENGER,MATTHIAS KRETZLER,MELISSA J. BURNE,HAMID RABB, and HUGH R. BRADY Human Genomics and Bioinformatics Research Unit, Department of Medicine and Therapeutics, Conway Institute of Biomolecular and Biomedical Research, University College Dublin, Mater Misericordiae Hospital, Dublin 7 and the Dublin Molecular Medicine Centre, Ireland; Medizinische Poliklinik, Ludwig-Maximilians-Universitat, Munchen, Germany; and Nephrology Division, Johns Hopkins University Hospital, Baltimore, MD, USA Modification of the transcriptomic response to renal ischemia/ (e.g., aquaporin-1) and the zinc metalloendopeptidase meprin- reperfusion injury by lipoxin analog. 1 implicated in renal remodeling. Background. Lipoxins are lipoxygenase-derived eicosanoids Conclusion. Treatment with the lipoxin analog 15-epi-16- with anti-inflammatory and proresolution bioactivities in vitro (FPhO)-LXA4-Me prior to injury modified the expression of and in vivo. We have previously demonstrated that the stable many differentially expressed pathogenic mediators, including synthetic LXA4 analog 15-epi-16-(FPhO)-LXA4-Me is reno- cytokines, growth factors, adhesion molecules, and proteases, protective in murine renal ischemia/reperfusion injury, as suggesting -

Functional and Structural Insights Into Astacin Metallopeptidases
Biol. Chem., Vol. 393, pp. 1027–1041, October 2012 • Copyright © by Walter de Gruyter • Berlin • Boston. DOI 10.1515/hsz-2012-0149 Review Functional and structural insights into astacin metallopeptidases F. Xavier Gomis-R ü th 1, *, Sergio Trillo-Muyo 1 Keywords: bone morphogenetic protein; catalytic domain; and Walter St ö cker 2, * meprin; metzincin; tolloid; zinc metallopeptidase. 1 Proteolysis Lab , Molecular Biology Institute of Barcelona, CSIC, Barcelona Science Park, Helix Building, c/Baldiri Reixac, 15-21, E-08028 Barcelona , Spain Introduction: a short historical background 2 Institute of Zoology , Cell and Matrix Biology, Johannes Gutenberg University, Johannes-von-M ü ller-Weg 6, The fi rst report on the digestive protease astacin from the D-55128 Mainz , Germany European freshwater crayfi sh, Astacus astacus L. – then termed ‘ crayfi sh small-molecule protease ’ or ‘ Astacus pro- * Corresponding authors tease ’ – dates back to the late 1960s (Sonneborn et al. , 1969 ). e-mail: [email protected]; [email protected] Protein sequencing by Zwilling and co-workers in the 1980s did not reveal homology to any other protein (Titani et al. , Abstract 1987 ). Shortly after, the enzyme was identifi ed as a zinc met- allopeptidase (St ö cker et al., 1988 ), and other family mem- The astacins are a family of multi-domain metallopepti- bers emerged. The fi rst of these was bone morphogenetic β dases with manifold functions in metabolism. They are protein 1 (BMP1), a protease co-purifi ed with TGF -like either secreted or membrane-anchored and are regulated growth factors termed bone morphogenetic proteins due by being synthesized as inactive zymogens and also by co- to their capacity to induce ectopic bone formation in mice localizing protein inhibitors. -

Genomic and Proteomic Analyses of the Coral Pathogen Vibrio Coralliilyticus Reveal a Diverse Virulence Repertoire
The ISME Journal (2011) 5, 1471–1483 & 2011 International Society for Microbial Ecology All rights reserved 1751-7362/11 www.nature.com/ismej ORIGINAL ARTICLE Genomic and proteomic analyses of the coral pathogen Vibrio coralliilyticus reveal a diverse virulence repertoire Eidy de O Santos1, Nelson Alves Jr1, Graciela M Dias1, Ana Maria Mazotto2, Alane Vermelho2, Gary J Vora3, Bryan Wilson4, Victor H Beltran4, David G Bourne4, Fre´de´rique Le Roux5,6 and Fabiano L Thompson1 1Laboratory of Microbiology, Institute of Biology, Federal University of Rio de Janeiro (UFRJ), Rio de Janeiro, Brazil; 2Laboratory of Proteases, Institute of Microbiology, UFRJ, Rio de Janeiro, Brazil; 3Center for Bio/ Molecular Science & Engineering, Naval Research Laboratory, Washington, DC, USA; 4Centre for Marine Microbiology and Genetics, Australian Institute of Marine Science, Townsville MC, Queensland, Australia; 5FR2424 CNRS UPMC Station Biologique de Roscoff, France and 6Ifremer, Laboratoire de Physiologie des Inverte´bre´s, Brest, France Vibrio coralliilyticus has been implicated as an important pathogen of coral species worldwide. In this study, the nearly complete genome of Vibrio coralliilyticus strain P1 (LMG23696) was sequenced and proteases implicated in virulence of the strain were specifically investigated. The genome sequence of P1 (5 513 256 bp in size) consisted of 5222 coding sequences and 58 RNA genes (53 tRNAs and at least 5 rRNAs). Seventeen metalloprotease and effector (vgrG, hlyA and hcp) genes were identified in the genome and expressed proteases were also detected in the secretome of P1. As the VcpA zinc-metalloprotease has been considered an important virulence factor of V. coralliilyticus, a vcpA deletion mutant was constructed to evaluate the effect of this gene in animal pathogenesis.