Multipole Expansion
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ph501 Electrodynamics Problem Set 1
Princeton University Ph501 Electrodynamics Problem Set 1 Kirk T. McDonald (1998) [email protected] http://physics.princeton.edu/~mcdonald/examples/ References: R. Becker, Electromagnetic Fields and Interactions (Dover Publications, New York, 1982). D.J. Griffiths, Introductions to Electrodynamics, 3rd ed. (Prentice Hall, Upper Saddle River, NJ, 1999). J.D. Jackson, Classical Electrodynamics, 3rd ed. (Wiley, New York, 1999). The classic is, of course: J.C. Maxwell, A Treatise on Electricity and Magnetism (Dover, New York, 1954). For greater detail: L.D. Landau and E.M. Lifshitz, Classical Theory of Fields, 4th ed. (Butterworth- Heineman, Oxford, 1975); Electrodynamics of Continuous Media, 2nd ed. (Butterworth- Heineman, Oxford, 1984). N.N. Lebedev, I.P. Skalskaya and Y.S. Ulfand, Worked Problems in Applied Mathematics (Dover, New York, 1979). W.R. Smythe, Static and Dynamic Electricity, 3rd ed. (McGraw-Hill, New York, 1968). J.A. Stratton, Electromagnetic Theory (McGraw-Hill, New York, 1941). Excellent introductions: R.P. Feynman, R.B. Leighton and M. Sands, The Feynman Lectures on Physics,Vol.2 (Addison-Wesely, Reading, MA, 1964). E.M. Purcell, Electricity and Magnetism, 2nd ed. (McGraw-Hill, New York, 1984). History: B.J. Hunt, The Maxwellians (Cornell U Press, Ithaca, 1991). E. Whittaker, A History of the Theories of Aether and Electricity (Dover, New York, 1989). Online E&M Courses: http://www.ece.rutgers.edu/~orfanidi/ewa/ http://farside.ph.utexas.edu/teaching/jk1/jk1.html Princeton University 1998 Ph501 Set 1, Problem 1 1 1. (a) Show that the mean value of the potential over a spherical surface is equal to the potential at the center, provided that no charge is contained within the sphere. -

Multipole Expansion, S.C. Magnets
!*(9:7*=/ 2. Charged particles in magnetic fields 2.2 Magnets (synchrotron magnet) 2.3 Multipole expansion 2.4 Superconducting magents Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 1 ,_,=+&,3*98 (42'.3*)=+:3(9.43a=8>3(-749743=2&,3*9 Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 2 942'.3*)=+:3(9.43=2&,3*9a=8>3(-749743=2&,3*9 Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 3 ,_-=+:19.541* *=5&38.43= Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 4 >*3*7&1=2:19.541* *=5&38.43 Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 5 >*3*7&1=2:19.541* *=5&38.43a=9>1.3)7.(&1= (447).3&9*=7*57*8*39&9.43=@ Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 6 >*3*7&1=2:19.541* *=5&38.43a=9>1.3)7.(&1= (447).3&9*=7*57*8*39&9.43=@@ Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 7 >*3*7&1=2:19.541* *=5&38.43a=9>1.3)7.(&1= (447).3&9*=7*57*8*39&9.43=@@@ Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 8 >*3*7&1=2:19.541* *=5&38.43a=9>1.3)7.(&1= (447).3&9*=7*57*8*39&9.43=@B Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 9 >*3*7&1=2:19.541* *=5&38.43a=9&79*8.&3=(447).3&9*= 7*57*8*39&9.43=@ Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 12 >*3*7&1=2:19.541* *=5&38.43a=9&79*8.&3=(447).3&9*= 7*57*8*39&9.43=@@ Matthias Liepe, P4456/7656, Spring 2010, Cornell University Slide 13 C.*1)=).897.':9.43=4+=9-*=+.789=+*<=2:19.541*8 Matthias Liepe, P4456/7656, Spring 2010, Cornell University -

Multipole Expansion for Radiation;Vector Spherical Harmonics
Multipole Expansion for Radiation;Vector Spherical Harmonics Physics 214 2013, Electricity and Magnetism Michael Dine Department of Physics University of California, Santa Cruz February 2013 Physics 214 2013, Electricity and Magnetism Multipole Expansion for Radiation;Vector Spherical Harmonics We seek a more systematic treatment of the multipole expansion for radiation. The strategy will be to consider three regions: 1 Radiation zone: r λ d. This is the region we have already considered for the dipole radiation. But we will see that there is a deeper connection between the usual multipole moments and the radiation at large distances (which in all cases falls as 1=r). 2 Intermediate zone (static zone): λ r d: Note that time derivatives are of order 1/λ (c = 1), while derivatives with respect to r are of order 1=r, so in this region time derivatives are negligible, and the fields appear static. Here we can do a conventional multipole expansion. 3 Near zone: d r. Here is is more difficult to find simple approximations for the fields. Physics 214 2013, Electricity and Magnetism Multipole Expansion for Radiation;Vector Spherical Harmonics Our goal is to match the solutions in the intermediate and radiation zones. We will see that in the intermediate zone, because of the static nature of the field, there is a multipole expansion identical to that of electrostatics (where moments are evaluated at each instant). This solution will match onto eikr−i!t outgoing spherical waves, all falling as r , but with a sequence of terms suppressed by powers of d/λ. So we have two kinds of expansion going on, a different one in each region. -
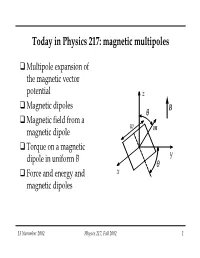
Today in Physics 217: Magnetic Multipoles
Today in Physics 217: magnetic multipoles Multipole expansion of the magnetic vector potential z Magnetic dipoles θ B Magnetic field from a w magnetic dipole m Torque on a magnetic y dipole in uniform B θ Force and energy and x magnetic dipoles 13 November 2002 Physics 217, Fall 2002 1 Multipole expansion of the magnetic vector potential Consider an arbitrary loop that carries a current I. Its vector potential at point r is r Id r =−rr′ Ar()= . θ c ∫ r Just as we did for V, we can expand 1 r I r’ in a power series and use the series as an approximation scheme: d ∞ n 11 r′ = ∑ Pn ()cosθ r rrn=0 (see lecture notes for 21 October 2002 for derivation). 13 November 2002 Physics 217, Fall 2002 2 Multipole expansion of the magnetic vector potential (continued) Put this series into the expression for A: I 11 Ar()=+ drd′cosθ Monopole, dipole, cr ∫∫r2 1322 1 +−rd′ cos θ quadrupole, r3 ∫ 22 1533 3 +−rd′ cosθθ cos octupole r4 ∫ 22 +… Of special note in this expression: 13 November 2002 Physics 217, Fall 2002 3 Multipole expansion of the magnetic vector potential (continued) The monopole term is zero, since ∫ d = 0. This isn’t surprising, since “no magnetic monopoles” is built into the Biot-Savart law, from which we obtained A. For points far away from the loop compared to its size, we obtain a good approximation for A by using just the first (or first two) nonvanishing terms. (For points closer by, one would need more terms for the same accuracy.) This is, of course, the same useful behaviour we saw in the multipole expansion of V. -

Multipole Expansions in Radiation Theory of Quantum Systems
Multipole expansions in quantum radiation theory M. Ya. Agre National University of “Kyiv-Mohyla Academy”, 04655 Kyiv, Ukraine E-mail: [email protected] With the help of mathematical technique of irreducible tensors the multipole expansion for the probability amplitude of spontaneous radiation of a quantum system is derived. It is shown that the found series represents the total radiation amplitude in the form of the sum of radiation amplitudes of electric and magnetic 2l-pole (l = 1, 2, 3,…) photons. All information about the radiating system is contained in the coefficients of the series which are the irreducible tensors being determined by the current density of transition. The expansion can be used for solving different problems that arise in studying electromagnetic field-quantum system interaction both in long-wave approximation and outside its framework. PACS number(s): 31.15.ag, 32.70.-n, 32.90.+a I. INTRODUCTION aligned (i.e., spin-polarized) atoms in long-wave 1 approximation . The known multipole expansion for Multipole expansions in classical electrodynamics the probability amplitude of a quantum transition are the seriess for potentials or field strengths, where accompanied by radiation of photon with definite the coefficients are the irreducible tensors dependent polarization and direction of motion is based on on the charge or current distributions of the system that representation of function e expik r, where e is is the source of the field. In every term of the multipole the vector of right(left)-hand circular polarization and k series the irreducible tensors have been convolved in is the wave vector (i.e., the momentum of the photon in scalars (for the scalar potential) or vectors (for the the units of ), in the form of the series in the vector potential or field strength) with the irreducible spherical vectors or the fields of electric and magnetic tensors specifying the corresponding fields (called the multipoles (see, e.g., [1]). -

Problem 1. Practice with Delta-Fcns a Delta-Function Is a Infinitely Narrow Spike with Unit Integral
Problem 1. Practice with delta-fcns A delta-function is a infinitely narrow spike with unit integral. R dx δ(x) = 1. (a) (Optional). A theta function (or step function) is 8 1 x > x <> o θ(x − xo) = 0 x < xo (1) :> 1 2 x = xo Not worrying about the case when x = xo, show that d θ(x − x ) = δ(x − x ) (2) dx o o (b) (Optional) Show that 1 δ(ax) = δ(x) (3) jaj (c) (Optional) Using the identity of part (b), show that X 1 δ(g(x)) = δ(x − x ) where g(x ) = 0 and g0 (x ) 6= 0 (4) jg0(x )j m m m m m m (d) Show that Z 1 1 dx δ(cos(x)) e−x = (5) 0 2 sinh(π=2) The delta function δ(x) should be thought of as sequence of functions δ(x) { known as a Dirac sequence { which becomes infinitely narrow and have integral one. For example, an infinitely narrow sequence of normalized Gaussians 1 x2 − 2 δ(x) = lim δ(x) = lim p e 2 : (6) !0 !0 2π2 The important properties are Z 1 = dx δ(x) (7) and the convolution property Z f(x) = lim dxof(xo)δ(x − xo) (8) !0 I will notate any Dirac sequence with δ(x). Delta functions are perhaps best thought about in Fourier space. In particular think about Eq. (??) in Fourier space. At finite epsilon this reads f(k) ' f(k)δ(k) : (9) 1 So the Fourier transform of a Dirac sequence δ(k) should be essentially one, except at large k where the function f(k) is presumably small. -

Closed Form Expressions for Gravitational Multipole Moments of Elementary Solids
Closed form expressions for gravitational multipole moments of elementary solids Julian Stirling1;∗ and Stephan Schlamminger2 1 Department of Physics, University of Bath, Claverton Down, Bath, BA2 7AY, UK 2 National Institute of Standards and Technology, 100 Bureau Drive, Gaithersburg, MD 20899, USA October 1, 2019 Abstract Perhaps the most powerful method for deriving the Newtonian gravitational interaction between two masses is the multipole expansion. Once inner multipoles are calculated for a particular shape this shape can be rotated, translated, and even converted to an outer multipole with well established methods. The most difficult stage of the multipole expansion is generating the initial inner multipole moments without resorting to three dimensional numerical integration of complex functions. Previous work has produced expressions for the low degree inner multipoles for certain elementary solids. This work goes further by presenting closed form expressions for all degrees and orders. A combination of these solids, combined with the aforementioned multipole transformations, can be used to model the complex structures often used in precision gravitation experiments. 1 Introduction In the field of precision gravitational measurements, the measurements and its associated analysis are often only half of the battle in producing a result. The other half comes from computing the theoretical Newtonian gravitational interaction for comparison. Computation of gravitational fields, forces, and torques can be accomplished by calculating sextuple integrals over the volumes arXiv:1707.01577v2 [gr-qc] 30 Sep 2019 of mass pairs, and summing for all pairs of source and test masses. Even with advanced methods to reduce these sextuple integrals to quadruple integrals [1, 2], for certain elementary solids, this is extremely computationally intensive, especially considering that for many measurements this needs to be entirely recalculated for multiple source mass positions. -
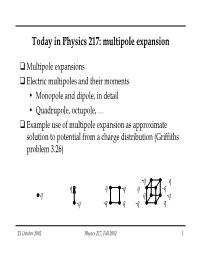
Today in Physics 217: Multipole Expansion
Today in Physics 217: multipole expansion Multipole expansions Electric multipoles and their moments • Monopole and dipole, in detail • Quadrupole, octupole, … Example use of multipole expansion as approximate solution to potential from a charge distribution (Griffiths problem 3.26) -q q q q -q q -q q q -q -q -q q -q q 21 October 2002 Physics 217, Fall 2002 1 Solving the Laplace and Poisson equations by sleight of hand The guaranteed uniqueness of solutions has spawned several creative ways to solve the Laplace and Poisson equations for the electric potential. We will treat three of them in this class: Method of images (9 October). Very powerful technique for solving electrostatics problems involving charges and conductors. Separation of variables (11-18 October) Perhaps the most useful technique for solving partial differential equations. You’ll be using it frequently in quantum mechanics too. Multipole expansion (today) Fermi used to say, “When in doubt, expand in a power series.” This provides another fruitful way to approach problems not immediately accessible by other means. 21 October 2002 Physics 217, Fall 2002 2 Multipole expansions Suppose we have a known charge distribution for which we want to know the potential or field outside the region where the charges are. If the distribution were symmetrical enough we could find the answer by several means: direct calculation using Gauss’ Law; direct calculation Coulomb’s Law; solution of the Laplace equation, using the charge distribution for boundary conditions. But even when ρ is symmetrical this can be a lot of work. Moreover, it may give more precise information on the potential or field than is actually needed. -

Spherical Tensor Approach to Multipole Expansions. I
Spherical tensor approach to multipole expansions. I. Electrostatic interaction~l~~ C. G. GRAY Department of Physics, University of Grtelph, Glrelpl~,Ontrrrio Received July 25, 1975 Using spherical harmonic expansions, the electrostatic field due to a given charge distribution, the interaction energy of a charge distribution with a given external field, and the electrostatic interaction energy of two charge distributions are decomposed into multipolar components. Extensive use is made of symmetry arguments. Comparisons with the Cartesian tensor method are also given. Au moyen de developpements en harmoniques spheriques, on obtient le champ d'une distribu- tion de charge donne. les composantes multipolaires de I'energie d'interaction d'une distribution de charge avec un champ exterieur donne, ainsi que de I'energie d'interaction electrostatique de deux distributions de charge. On fait un usage considerable des arguments de symetrie. On donne aussi des comparaisons avec la methode des tenseurs Cartksiens. [Traduit par le journal] Can. J. Phys.. 54,505 (1976) 1. Introduction In the next section we discuss the classic 'one- Multipole interactions have been reviewed a center' expansion problem, i.e. the decomposi- number of times in recent years in the Cartesian tion of the potential field due to a given charge tensor formalism (Buckingham 1965, 1970). Al- distribution into multipolar contributions. The though some individual topics have been dis- solution to this problem leads to a natural intro- cussed (Jackson 1962; Brink and Satchler 1962; duction of the spherical multipole components Rose 1957), there appears, however, to be no Q,,. We give the general properties of the Q,,, comprehensive review from the spherical har- and special properties for charge distributions of For personal use only. -

Multipole Expansion for Magnetic Structures: a Generation Scheme for a Symmetry-Adapted Orthonormal Basis Set in the Crystallographic Point Group
PHYSICAL REVIEW B 99, 174407 (2019) Editors’ Suggestion Multipole expansion for magnetic structures: A generation scheme for a symmetry-adapted orthonormal basis set in the crystallographic point group M.-T. Suzuki,1 T. Nomoto,2 R. Arita,2,3 Y. Yanagi,1 S. Hayami,4 and H. Kusunose5 1Center for Computational Materials Science, Institute for Materials Research, Tohoku University, Sendai, Miyagi 980-8577, Japan 2Department of Applied Physics, University of Tokyo, 7-3-1 Hongo Bunkyo-ku, Tokyo 113-8656, Japan 3RIKEN Center for Emergent Matter Science (CEMS), Wako, Saitama 351-0198, Japan 4Department of Physics, Hokkaido University, Sapporo 060-0810, Japan 5Department of Physics, Meiji University, Kawasaki 214-8571, Japan (Received 1 March 2019; published 10 May 2019) We propose a systematic method to generate a complete orthonormal basis set of multipole expansion for magnetic structures in arbitrary crystal structure. The key idea is the introduction of a virtual atomic cluster of a target crystal on which we can clearly define the magnetic configurations corresponding to symmetry-adapted multipole moments. The magnetic configurations are then mapped onto the crystal so as to preserve the magnetic point group of the multipole moments, leading to the magnetic structures classified according to the irreducible representations of the crystallographic point group. We apply the present scheme to pyrochlore and hexagonal ABO3 crystal structures and demonstrate that the multipole expansion is useful to investigate the macroscopic responses of antiferromagnets. DOI: 10.1103/PhysRevB.99.174407 I. INTRODUCTION characterize the specific magnetic structures [10–12,17]. The relation between parity odd multipoles and electromagnetic Diversity of physical properties of magnets provides a effect has recently been investigated based on generalized fascinating playground in condensed matter physics. -

Multipole Expansions
Multipole Expansions Recommended as prerequisites Vector Calculus Coordinate systems Separation of PDEs - Laplace’s equation Concepts of primary interest: Consistent expansions and small parameters Multipole moments Monopole moment Dipole moment Quadrupole moment Sample calculations: Decomposition example Quadrupole expansion with details To Add: Energy of a distribution in an external potential Using Symmetry to Avoid Calculations Tools of the trade: Taylor’s series expansions in three dimensions Relation to the Legendre Expansion in Griffiths Using Symmetry to Avoid Calculations A multipole expansion provides a set of parameters that characterize the potential due to a charge distribution of finite size at large distances from that distribution. The goal is to represent the potential by a series expansion of the form: [email protected] Vr() V012 () r Vr () V () r ... [MP.1] total = zeroth order + first order + second order + … n ('Coulomb s Constant )charge size of the distribution where each term Vrn () is proportional to distance distance{} to field point . size of the distribution typicallinear dimension The small expansion parameter is distance{} to field point or distance{ to field point} and n is the order of the term in the small parameter. The Coulomb integral provides an exact expression for the electrostatic potential at points in space. ()rdr 3 Vr() 1 [MP.2] 40 rr The symbol d3r' represents a volume element for the source (primed) coordinates, r is the position vector for the source charge and r is the position vector for the field point at which the potential is to be computed. The equation directs that a "sum" over the source charges be computed. -

Multipole Expansion (Potentials at Large Distances)
Semester II, 2017-18 Department of Physics, IIT Kanpur PHY103A: Lecture # 11 (Text Book: Intro to Electrodynamics by Griffiths, 3rd Ed.) Anand Kumar Jha 24-Jan-2018 Notes • No Class on Friday; No office hour. • HW # 4 has been posted • Solutions to HW # 5 have been posted 2 Summary of Lecture # 10: The potential in the region above the infinite grounded conducting plane? 1 1 V = x + y + (z d) x + y + (z + d) 0 2 2 2 − 2 2 2 4 − The potential outside the grounded conducting sphere? = = 2 ′ − 1 V = + r r′ ′ 0 4 3 Question: The potential in the region above the infinite grounded conducting plane? 1 1 V = x + y + (z d) x + y + (z + d) 0 2 2 2 − 2 2 2 4 − Q: Is kV also a solution, where k is a constant? Ans: No Corollary to First Uniqueness Theorem: The potential in a volume is uniquely determined if (a) the charge desity throughout the region and (b) the value of V at all boundaries, are specified. In this case one has to satisfy the Poisson’s V = equation. But kV does not satisfy it. 2 1 − 0 Exception: Only when = 0 (everywhere), kV can be a solution as well But then since in this case V = , it is a trivial solution. 4 Multipole Expansion (Potentials at large distances) • What is the potential due to a point charge (monopole)? 1 V = (goes like / ) • What is the potential4 due0 to a dipole at large distance? 1 V = r r − 40 + − r = + cos = 1 cos + ± 2 2 4 2 2 2 2 ∓ ∓ 2 1 cos (for ) 2 for , and using binomial≈ expansion ∓ ≫ 1 1 1 1 1 1 ≫= 1 cos − 1 ± cos So, = cos r± 2 r r ∓ 1 ≈ cos − 2 + − V = 2 (goes like / at large ) 5 2 40 Multipole Expansion (Potentials at large distances) • What is the potential due to a quadrupole at large distance? (goes like / at large ) • What is the potential due to a octopole at large distance? (goes like / at large ) Note1: Multipole terms are defined in terms of their dependence, not in terms of the number of charges.