Lethality of Honey Bee Stings to Heavily Armored Hornets
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Geographic Variation in the Japanese Islands of Apis Cerana Japonica and in A
Apidologie 38 (2007) 335–340 Available online at: c INRA/DIB-AGIB/ EDP Sciences, 2007 www.apidologie.org DOI: 10.1051/apido:2007018 Original article Geographic variation in the Japanese islands of Apis cerana japonica and in A. cerana populations bordering its geographic range* Jun-ichi Ta, Tadaharu Ya, Toshiyuki Tb, Shin’ichi Ac, Kun S. Wd, Sureerat De, Randall Hf,JunNa, Mitsuo M a a Honeybee Science Research Center, Research Institute, Tamagawa University, Machida, Tokyo, 194-8610, Japan b Laboratory of Entomology, Department of Agriculture, Graduate School of Agriculture, Tamagawa University, Machida, Tokyo, 194-8610, Japan c Laboratory of Systematic Entomology, Department of Ecology and Systematics, Graduate School of Agriculture, Hokkaido University, Sapporo, 060-8589, Japan d Institute of Korea Beekeeping Science College of Agriculture and Life Sciences, Seoul National University e Bee Biology Research Unit, Department of Biology, Chulalongkom University, Korea, Bangkok 10330, Thailand f Department of Zoology and Entomology, Rhodes University, Grahamstown 6140, South Africa Received 31 January 2006 – Revised 15 February 2007 – Accepted 15 February 2007 Abstract – Genetic variation among Apis cerana japonica isolates from Japan and Apis cerana isolates from the neighboring areas of Russia, South Korea, and Taiwan was determined from DNA sequences of the mitochondrial DNA non-coding region (between tRNA leu and COII). Three haplotypes were identified among 470 colonies samples at 47 Japanese sites. All isolates from the main Japanese Islands of Honshu, Shikoku, and Kyushu belonged to a single haplotype, a previously reported Japan 1 haplotype. Two new haplotypes were found on the far southern Japanese islands of Amami-Oshima and Tsushima (the Japan 3 and Japan 4 haplotypes, respectively). -

Ecology, Behaviour and Control of Apis Cerana with a Focus on Relevance to the Australian Incursion
Insects 2013, 4, 558-592; doi:10.3390/insects4040558 OPEN ACCESS insects ISSN 2075-4450 www.mdpi.com/journal/insects/ Review Ecology, Behaviour and Control of Apis cerana with a Focus on Relevance to the Australian Incursion Anna H. Koetz Biosecurity Queensland, Department of Agriculture, Fisheries and Forestry, 21-23 Redden St., Portsmith, QLD 4870, Australia; E-Mail: [email protected]; Tel.: +61-419-726-698; Fax: +61-7-4057-3690 Received: 27 June 2013; in revised form: 13 September 2013 / Accepted: 24 September 2013 / Published: 21 October 2013 Abstract: Apis cerana Fabricius is endemic to most of Asia, where it has been used for honey production and pollination services for thousands of years. Since the 1980s, A. cerana has been introduced to areas outside its natural range (namely New Guinea, the Solomon Islands, and Australia), which sparked fears that it may become a pest species that could compete with, and negatively affect, native Australian fauna and flora, as well as commercially kept A. mellifera and commercial crops. This literature review is a response to these concerns and reviews what is known about the ecology and behaviour of A. cerana. Differences between temperate and tropical strains of A. cerana are reviewed, as are A. cerana pollination, competition between A. cerana and A. mellifera, and the impact and control strategies of introduced A. cerana, with a particular focus on gaps of current knowledge. Keywords: Apis cerana; Apis mellifera; incursion; pest species; Australia; pollination; competition; distribution; control 1. Introduction Apis cerana Fabricius (also known as the Asian honeybee, Asiatic bee, Asian hive bee, Indian honeybee, Indian bee, Chinese bee, Mee bee, Eastern honeybee, and Fly Bee) is endemic to most of Asia where it has been used for honey production and pollination services for thousands of years. -

Management of Insect Sting Hypersensitivity: an Update Robert D
Review Allergy Asthma Immunol Res. 2013 May;5(3):129-137. http://dx.doi.org/10.4168/aair.2013.5.3.129 pISSN 2092-7355 • eISSN 2092-7363 Management of Insect Sting Hypersensitivity: An Update Robert D. Pesek,1* Richard F. Lockey2 1Division of Allergy and Immunology, Arkansas Children’s Hospital, Little Rock, AR, USA 2Division of Allergy and Immunology, University of South Florida and the James A. Haley Veterans’ Hospital, Tampa, FL, USA This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Reactions to Hymenoptera insect stings are common. While most are self-limited, some induce systemic allergic reactions or anaphylaxis. Prompt recognition, diagnosis, and treatment of these reactions are important for improving quality-of-life and reducing the risk of future sting reactions. This review summarizes the current recommendations to diagnose and treat Hymenoptera sting induced allergic reactions and highlights considerations for various populations throughout the world. Key Words: Hymenoptera allergy; venom immunotherapy; sting-induced anaphylaxis; insect sting allergy; insect sting hypersensitivity INTRODUCTION (Polistes); family Apidea (bees); and family Formicidae (ants) (Figure).3 Proper recognition of the insect responsible for the Allergic reactions triggered by Hymenoptera insects have -

Comparative Performance of Apis Mellifera and Apis Cerana Under Punjab Conditions
Volume : 4 | Issue : 3 | Mar 2015 ISSN - 2250-1991 Research Paper Medical Science Comparative Performance of Apis Mellifera and Apis Cerana Under Punjab Conditions JASVIR SINGH DALIO Street No. 12, Yog Nagar BUDHLADA-151502 (PUNJAB) Study conducted on relative performance of Apis mellifera and A. cerana under Punjab conditions, revealed that as far as honey collection, pollen load, egg laying capacity, sustainability under adverse conditions (dearth period), ability to regain strength after deteriorating environmental conditions etc. were concerned, A. mellifera was the best performer as compared to other species. Phenomena of absconding and swarming was more in case of A. cerana while absconding was not observed and swarming was easily controllable in case of A. mellifera. Thus Italian honeybees were more suitable and ABSTRACT beneficial as compared to A. cerana. KEYWORDS Apis mellifera, Apis cerana, swarming, absconding, foraging behaviour of honeybees. Introduction A. mellifera queen was much higher than that of A. cerana Biology of Apis mellifera and A. cerana is similar in many (Table-1). ways. Both types make parallel combs in dark. Performance of these honeybee species may differ in different geographical Swarming and absconding took place more frequently in A. areas and various agro-ecosystems. Mostly A. cerana is reared cerana. No absconding was recorded in A. mellifera even dur- in hilly areas whereas A. mellifera in plains. The former species ing dearth period (May to July). Average 45 per cent colonies starts foraging in early hours (morning) at low temperature absconded while 21 per cent colonies dwindled in case of A. in winter as compared to the latter one. -

Instruction Sheet: Bee Sting, Local Reaction
University of North Carolina Wilmington Abrons Student Health Center INSTRUCTION SHEET: BEE STING, LOCAL REACTION The Student Health Provider has diagnosed a mild allergic reaction to a bee/wasp sting. Fortunately, most bee stings are not serious and cause only temporary swelling, redness, and pain at the sting site. Rarely, a whole-body allergic reaction occurs; shock can result. The stinger, if still in the wound, should be removed; if the stinger is left in place, bee toxin continues to enter the body, increasing the reaction. A stinger should be removed with a piece of paper or credit card, using a sideways scraping motion. A pair of tweezers can also be used to remove the stinger, but try not to squeeze the stinger, or more toxin can be pushed inside the wound. Realize that swelling may increase at first, even with treatment. Measures can be taken, however, to minimize the reaction to bee stings. MEASURES YOU SHOULD TAKE TO HELP TREAT YOUR BEE STING: 1. Rest and elevate the affected body part. Rest and elevation help reduce swelling and pain. 2. Apply cold packs to the area off-and-on for the first 24 hours after injury. Cold helps ease discomfort, and minimizes additional swelling. Do not apply ice directly to the area, causing discomfort. Rather, aim for coolness, yet comfort, applying a layer or two of cloth between the cold pack and affected area. 3. Take over-the-counter antihistamines: In the morning, take a non-sedating antihistamine such as loratadine, 10 mg daily. At night, take diphenhydramine (Benadryl), 25 mg, 1 or 2 every 6 hours for itching and swelling. -

Bee Stings and Your Pets and Livestock
TREATING YOUR ANIMAL LOOK FOR STINGERS once the animal is away from bees. When a honey bee stings, it loses its venom sac and stinger. This means the honey bee dies after it stings, but the stinger may continue to in- ject venom for up to a minute or until the stinger is removed. If you can see stingers on the animal, remove then by scraping them out with a credit card, knife or finger- nail. Do not pull them out with tweezers or BEE STINGS AND fingers because you will squeeze more venom into the sting. YOUR PETS AND IF AN ANIMAL HAS SUSTAINED NU- LIVESTOCK MEROUS STINGS, EMERGENCY TREATMENT BY YOUR VETERINARIAN MAY BE REQUIRED. The number of stings an animal can survive depends on its body weight, the amount of venom it received and whether or not it is allergic to bee venom. As with humans, even one sting may be dangerous if the animal is allergic. Georgia Department of Agriculture Plant Protection Division Atlanta, Georgia 30334 Atlanta: 404-651-9486 Tifton: 229-386-3464 Africanized honey bees (AHB), some- Protecting Animals KEEP DOGS UNDER CONTROL times called “killer bees,” became es- WHEN HIKING. A dog bounding tablished in Texas in 1990 and are MAKE A REGULAR INSPECTION OF YOUR PROPERTY FOR BEE NESTS. through the brush is more likely to dis- spreading to other states including turb bees than one following quietly at Georgia. Honey bees nest in a wide variety of sites, such as trees or shrubs, animal your heels. The Africanized honey bee is related to burrows in the ground, water meter our state’s familiar honey bee, the Eu- boxes and overturned flower pots. -

Asian Giant Hornet in Washington State
ASIAN GIANT HORNET IN WASHINGTON STATE PEST PROGRAM INTRODUCTION ASIAN Asian giant hornet (Vespa mandarinia) is the world’s largest hornet. The hornet is native to Asia, and has GIANT been recorded from Japan, Korea, Russia, China, and several other countries. In December 2019, WSDA HORNET verified the first ever sightings of Asian giant hornet in the United States. If Asian giant hornet becomes Asian giant hornet (AGH) is a predatory wasp that established, it could feeds on a wide variety of insects. The introduction have serious impacts of this species is of major concern to agriculture on the environment, because of its predation on honeybees - a few Asian economy, and giant hornets can kill an entire beehive in a matter public health of of hours. If unmanaged they could significantly Washington State. increase costs for beekeepers and potentially disrupt pollination services. They could also impact other local insect populations. While AGH does not generally attack people or pets, they can sting when threatened. If it becomes established, this hornet could have serious impacts on the environment, economy, and public health of Washington State. Some of the Asian giant hornet specimens WSDA recovered during eradication of a nest in Blaine, WA - the first ever nest found in the U.S. ASIAN GIANT HORNET | 1 identification • Usually 1.5 - 2 inches in length, with queens being substantially larger than workers or males • Large orange/yellow head with prominent eyes • Black and orange/yellow striped abdomen • Forms large colonies that usually nest in the ground, although sometimes in tree cavities lookalikes • Western cicada killers are mostly rust-orange colored and have yellow spots on the abdomen. -

Visitation and Gnawing Behaviour of Japanese Honeybee Apis Cerana Japonica to Lettuce Tomoyuki Yokoi
Visitation and gnawing behaviour of Japanese honeybee Apis cerana japonica to lettuce Tomoyuki Yokoi To cite this version: Tomoyuki Yokoi. Visitation and gnawing behaviour of Japanese honeybee Apis cerana japonica to lettuce. Apidologie, Springer Verlag, 2015, 46 (4), pp.489-494. 10.1007/s13592-014-0340-z. hal- 01284460 HAL Id: hal-01284460 https://hal.archives-ouvertes.fr/hal-01284460 Submitted on 7 Mar 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie (2015) 46:489–494 Original article * INRA, DIB and Springer-Verlag France, 2014 DOI: 10.1007/s13592-014-0340-z Visitation and gnawing behaviour of Japanese honeybee Apis cerana japonica to lettuce Tomoyuki YOKOI Laboratory of Conservation Ecology, Faculty of Life and Environmental Sciences, University of Tsukuba, 1-1-1 Tennodai, Tsukuba City, Ibaraki 305-8572, Japan Received 1 August 2014 – Revised 13 November 2014 – Accepted 28 November 2014 Abstract – The Japanese honeybee, Apis cerana japonica , is regarded as an important pollinator of crops and wild flowers. On the other hand, its gnawing behaviour on lettuce Lactuca spp. results in serious damage to the crop yield. Little is known about the gnawing behaviour of A. -

The Armenians
THE ARMENIANS By C.F. DIXON-JOHNSON “Whosoever does wrong to a Christian or a Jew shall find me his accuser on the day of judgment.” (EL KORAN) Printed and Published by GEO TOULMIN & SONS, LTD. Northgate, Blackburn. 1916 Preface The following pages were first read as a paper before the “Société d’Etudes Ethnographiques.” They have since been amplified and are now being published at the request of a number of friends, who believe that the public should have an opportunity of judging whether or not “the Armenian Question” has another side than that which has been recently so assiduously promulgated throughout the Western World. Though the championship of Greek, Bulgarian and other similar “Christian, civilized methods of fighting,” as contrasted with “Moslem atrocities” in the Balkans and Asia Minor, has been so strenuously undertaken by Lord Bryce and others, the more recent developments in the Near East may perhaps already have opened the eyes of a great many thinking people to the realization that, in sacrificing the traditional friendship of the Turk to all this more or less sectarian clamor, British diplomacy has really done nothing better than to exchange the solid and advantageous reality for a most elusive and unreliable, if not positively dangerous, set of shadows. It seems illogical that the same party which recalled the officials (and among them our present War Minister) appointed by Lord Beaconsfield to assist the Turkish Government in reforming their administration and collecting the revenue in Asia Minor, and which on the advent of the Young Turks refused to lend British Administrators to whom ample and plenary powers were assured, should now, in its eagerness to vilify the Turk, lose sight of their own mistakes which have led in the main to the conditions of which it complains, and should so utterly condemn its own former policy. -

Bee Viruses: Routes of Infection in Hymenoptera
fmicb-11-00943 May 27, 2020 Time: 14:39 # 1 REVIEW published: 28 May 2020 doi: 10.3389/fmicb.2020.00943 Bee Viruses: Routes of Infection in Hymenoptera Orlando Yañez1,2*, Niels Piot3, Anne Dalmon4, Joachim R. de Miranda5, Panuwan Chantawannakul6,7, Delphine Panziera8,9, Esmaeil Amiri10,11, Guy Smagghe3, Declan Schroeder12,13 and Nor Chejanovsky14* 1 Institute of Bee Health, Vetsuisse Faculty, University of Bern, Bern, Switzerland, 2 Agroscope, Swiss Bee Research Centre, Bern, Switzerland, 3 Laboratory of Agrozoology, Department of Plants and Crops, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium, 4 INRAE, Unité de Recherche Abeilles et Environnement, Avignon, France, 5 Department of Ecology, Swedish University of Agricultural Sciences, Uppsala, Sweden, 6 Environmental Science Research Center, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand, 7 Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand, 8 General Zoology, Institute for Biology, Martin-Luther-University of Halle-Wittenberg, Halle (Saale), Germany, 9 Halle-Jena-Leipzig, German Centre for Integrative Biodiversity Research (iDiv), Leipzig, Germany, 10 Department of Biology, University of North Carolina at Greensboro, Greensboro, NC, United States, 11 Department Edited by: of Entomology and Plant Pathology, North Carolina State University, Raleigh, NC, United States, 12 Department of Veterinary Akio Adachi, Population Medicine, College of Veterinary Medicine, University of Minnesota, Saint Paul, MN, United States, -
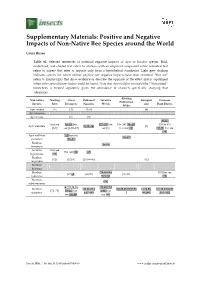
Positive and Negative Impacts of Non-Native Bee Species Around the World
Supplementary Materials: Positive and Negative Impacts of Non-Native Bee Species around the World Laura Russo Table S1. Selected references of potential negative impacts of Apis or Bombus species. Bold, underlined, and shaded text refers to citations with an empirical component while unbolded text refers to papers that refer to impacts only from a hypothetical standpoint. Light grey shading indicates species for which neither positive nor negative impacts have been recorded. “But see” refers to manuscripts that show evidence or describe the opposite of the effect and is capitalized when only contradictory studies could be found. Note that Apis mellifera scutellata (the “Africanized” honeybee), is treated separately given the abundance of research specifically studying that subspecies. Altering Non-native Nesting Floral Pathoens/ Invasive Introgres Decrease Pollination Species Sites Resources Parasites Weeds sion Plant Fitness Webs Apis cerana [1] [2] [1–3] [4] Apis dorsata Apis florea [5] [5] [37,45] But see [8–19] but [27–35] but [36–38] [39–43] [38,46,47] Apis mellifera [9,23–26] [4] [6,7] see [6,20–22] see [6] but see [44] [48,49] but see [50] Apis mellifera [51] but see [55–57] scutellata [52–54] Bombus [58,59] hortorum Bombus But see But see [60] [61] hypnorum [60] Bombus [62] [62,63] [26,64–66] [62] impatiens Bombus lucorum Bombus [28,58,59,6 [39] but see [67,68] [69,70] [36,39] ruderatus 9,71,72] [73] Bombus [59] subterraneous [67,70,74,75, [29,58,72,9 Bombus [25,26,70,7 [38,39,68,81,97,98 [4,76,88, [47,76,49,86,97 [74–76] 77–84] but 1–95] but terrestris 6,87–90] ] 99,100] ,101–103] see [85,86] see [96] Insects 2016, 7, 69; doi:10.3390/insects7040069 www.mdpi.com/journal/insects Insects 2016, 7, 69 S2 of S8 Table S2. -

How Things Fly Presentations
1A Crawford, Jasmine I Eagle 1A Elliott, Noah C Bumble Bee 1A Patel, Kishan H Hornet 1A Sutton, Ashley K Turkey 1B Ablorh, Marcellina A Birds 1B Bailey-Simpson, Tyron D Bumble Bee 1B Butler, Vidal M Hornet 1B Castor, Carnie Wasp 1B Clark, Micaylah J Butterfly 1B Fairley, Nicholas M Duck 1B Gaymer, Nicolas Falcon 1B Gomez, Luis G Eagle 1B Haines, Tynise N Geese 1B Harden, Travis E Ostrich 1B Henry, Nigel D Turkey 1B Jenkins, Myae'h J Birds 1B Johnson, Jeremy A Bumble Bee 1B Jordan, Justin A Hornet 1B Kovach, Alecia A Wasp 1B Marshall, Allison V Butterfly 1B Marshall, Alyssa M Duck 1B McLennon, Michael K Falcon 1B Milazzo, Matthew M Eagle 1B Okwuosa, Arinze A Geese 1B Smith, Chasity R Ostrich 1B Thomas, Jada L Turkey 1B Warren, Dierra L Birds 1B Weems, Destiny K Bumble Bee 2A Anderson, Jeremiah O Birds 2A Barrett, Dakota A Bumble Bee 2A Bates, Brooklyn J Hornet 2A Blue II, Neil Wasp 2A Brock, Devin A Butterfly 2A Clark, Jasmine M Duck 2A Coleman, Arrienna D Falcon 2A Fisher, M'Kayla M Eagle 2A Harris, Johnathan L Geese 2A Heintz, Michael H Ostrich 2A Hester, Jazlyn A Turkey 2A Hobbs, Kamilah D Birds 2A Jackson, Janise N Bumble Bee 2A Jenkins II, Harrison C Hornet 2A Jones, Lance K Wasp 2A Jones, Shicorreus L Butterfly 2A Logan, Tyshawn A Duck 2A Maduchem-Izundu, KachikwuluFalcon M 2A Matthews, Zackary G Eagle 2A Nguyen, Jimmy Geese 2A Norfleet, Nyhjae Q Ostrich 2A Paragon-singh, Andrew W Turkey 2A Parrish Brown, Jordan C Birds 2A Petty, Kristina A Bumble Bee 2A Pitchford, Colin C Hornet 2A Singleton, Isaiah D Wasp 2A Tucker, Cody D Butterfly