Immunology Pre-Matriculation Review Answers the Answers to the Questions Are Shown in Blue Text. 1. One Reason That Pathogenic M
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Megakaryocyte Growth and Platelet Production (Blood Platelet/Hematopoleflc Growth Factor/Thrombocyoena/B~Usfan) DAVID J
Proc. Natl. Acad. Sci. USA Vol. 91, pp. 11104-11108, November 1994 Physiology The purification of megapoietin: A physiological regulator of megakaryocyte growth and platelet production (blood platelet/hematopoleflc growth factor/thrombocyoena/b~usfan) DAVID J. KUTER*tt§, DAVID L. BEELER*, AND ROBERT D. ROSENBERGt1¶ *Hematology Unit, Massachusetts General Hospital, Boston, MA; tHarvard Medical School, Boston, MA; tDepartment of Biology, Massachusetts Institute of Technology, Cambridge, MA; and 'Department of Medicine, Beth Israel Hospital, Boston, MA Communicated by Laszlo Lorand, July 8, 1994 (receivedfor review June 3, 1994) ABSTRACT The circulating blood platelet is produced by To characterize this putative thrombopoietic factor, we the bone marrow megkarocyte. In response to a decrease in have analyzed the physiological relationship between the the platelet count, megryocytes increase in number and bone marrow megakaryocytes and the circulating platelets. ploidy. Although this feedback loop has long been thought to be We have found that the magnitude of the changes in the medlated by a circulating hematopoletic factor, no such factor number and ploidy of megakaryocytes was inversely and has been purified. Using a model ofthrombocytopenia in sheep, proportionally related to the circulating platelet mass (3) and we have identified an active substance called megapoetin, that megakaryocyte number and ploidy were therefore mark- which simulated an increase in the number and ploldy of ers of this feedback loop in vivo. We next developed a bone meg-karyocytes in bone marrow culture. Circulating levels of marrow assay in which increases in the number and ploidy of this factor could be quantified with this assay and were found megakaryocytes in vitro (6) were used to identify an active to be Inversely proportional to the platelet count of the sheep. -

Ch. 43 the Immune System
Ch. 43 The Immune System 1 Essential Questions: How does our immunity arise? How does our immune system work? 2 Overview of immunity: Two kinds of defense: A. innate immunity present at time of birth before exposed to pathogens nonspecific responses, broad in range skin and mucous membranes internal cellular and chemical defenses that get through skin macrophages, phagocytic cells B. acquired immunity (adaptive immunity)develops after exposure to specific microbes, abnormal body cells, foreign substances or toxins highly specific lymphocytes produce antibodies or directly destroy cells 3 Overview of Immune System nonspecific Specific 4 I. Innate immunity Nonspecific defenses A. First line of defense: skin, mucous membranes skin protects against chemical, mechanical, pathogenic, UV light damage mucous membranes line digestive, respiratory, and genitourinary tracts prevent pathogens by trapping in mucus *breaks in skin or mucous membranes = entryway for pathogen 5 secretions of skin also protect against microbes sebaceous and sweat glands keep pH at 35 stomach also secretes HCl (Hepatitis A virus can get past this) produce antimicrobial proteins (lysozyme) ex. in tears http://vrc.belfastinstitute.ac.uk/resources/skin/skin.htm 6 B. Second line of defense internal cellular and chemical defenses (still nonspecific) phagocytes produce antimicrobial proteins and initiate inflammation (helps limit spread of microbes) bind to receptor sites on microbe, then engulfs microbe, which fused with lysosome nitric oxide and poisons in lysosome can poison microbe lysozyme and other enzymes degrade the microbe *some bacteria have capsule that prevents attachment *some bacteria are resistant to lysozyme macrophages 7 Cellular Innate defenses Tolllike receptor (TLR) recognize fragments of molecules characteristic of a set of pathogens [Ex. -

White Blood Cells and Severe COVID-19: a Mendelian Randomization Study
Journal of Personalized Medicine Article White Blood Cells and Severe COVID-19: A Mendelian Randomization Study Yitang Sun 1 , Jingqi Zhou 1,2 and Kaixiong Ye 1,3,* 1 Department of Genetics, Franklin College of Arts and Sciences, University of Georgia, Athens, GA 30602, USA; [email protected] (Y.S.); [email protected] (J.Z.) 2 School of Public Health, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China 3 Institute of Bioinformatics, University of Georgia, Athens, GA 30602, USA * Correspondence: [email protected]; Tel.: +1-706-542-5898; Fax: +1-706-542-3910 Abstract: Increasing evidence shows that white blood cells are associated with the risk of coronavirus disease 2019 (COVID-19), but the direction and causality of this association are not clear. To evaluate the causal associations between various white blood cell traits and the COVID-19 susceptibility and severity, we conducted two-sample bidirectional Mendelian Randomization (MR) analyses with summary statistics from the largest and most recent genome-wide association studies. Our MR results indicated causal protective effects of higher basophil count, basophil percentage of white blood cells, and myeloid white blood cell count on severe COVID-19, with odds ratios (OR) per standard deviation increment of 0.75 (95% CI: 0.60–0.95), 0.70 (95% CI: 0.54–0.92), and 0.85 (95% CI: 0.73–0.98), respectively. Neither COVID-19 severity nor susceptibility was associated with white blood cell traits in our reverse MR results. Genetically predicted high basophil count, basophil percentage of white blood cells, and myeloid white blood cell count are associated with a lower risk of developing severe COVID-19. -

Pulmonary Megakaryocytes: "Missing Link" Between Cardiovascular and Respiratory Disease?
J Clin Pathol: first published as 10.1136/jcp.39.9.969 on 1 September 1986. Downloaded from J Clin Pathol 1986;39:969-976 Pulmonary megakaryocytes: "missing link" between cardiovascular and respiratory disease? G K SHARMA, I C TALBOT From the Department ofPathology, University ofLeicester, Leicester Royal Infirmary SUMMARY Pulmonary megakaryocytes were quantitated in a series of 30 consecutive hospital nec- ropsies using a two stage immunoperoxidase stain for factor VIII related antigen. In all 30 cases they were found with a mean density of 14 65 megakaryocytes/cm2 in lung sections of 5 gm in thickness. The maximum concentration of intrapulmonary megakaryocytes was consistently found to be in the central zone of the right upper lobe. Less than 22% of the observed cells possessed abundant cytoplasm, the rest appearing as effete, naked, and seminaked nuclei. The mean megakaryocyte count was found to be increased in association with both respiratory pathology (positive smoking history and impaired lung function) and cardiovascular disease states-shock; thromboembolism; myocardial infarction; and severe atheroma in the abdominal aorta, the coronary circulation, and the circle of Willis. Pulmonary megakaryocytes probably embolise from bone marrow. This may reflect stimulated thrombopoiesis, caused by increased platelet consumption in association with atherosclerotic disease, but it cannot be taken to confirm that the lung is the principal site of platelet production. copyright. The megakaryocyte is now firmly established as the 2 Intact megakaryocytes -

For Inflammatory Bowel Disease
250 NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedures overview of leukapheresis (white cell apheresis) for inflammatory bowel disease Introduction This overview has been prepared to assist members of the Interventional Procedures Advisory Committee (IPAC) in making recommendations about the safety and efficacy of an interventional procedure. It is based on a rapid review of the medical literature and specialist opinion. It should not be regarded as a definitive assessment of the procedure. Date prepared This overview was prepared in August 2004. Procedure names • Leukapheresis • White cell apheresis. • Leukocyte removal therapy. • Selective granulocyte and monocyte adsorption apheresis. • Leukocytapheresis. Specialty society • British Society of Gastroenterology. Description Indications Inflammatory bowel disease. Ulcerative colitis and Crohn’s disease are the most common forms of inflammatory bowel disease. Ulcerative colitis causes inflammation and ulceration of the rectum and sometimes the colon. Symptoms include bloody diarrhoea and rectal bleeding. Crohn’s disease usually causes inflammation and ulceration of the small and large intestines, but it can affect any part of the digestive tract. The main symptoms are abdominal pain, diarrhoea and weight loss. Both of these are chronic conditions, characterised by periods of clinical relapse and remission. The incidence of ulcerative colitis is around 10 to 20 per 100,000 per year in the UK and the incidence of Crohn’s disease is approximately 5 to 10 per 100,000 per year.1 Current treatment and alternatives Conservative treatments include dietary measures, and medications to control inflammation. Immunosuppressants may be used if other medical therapies are ineffective at maintaining remission. Patients with ulcerative colitis that does not respond to medical therapy may be treated with surgery to remove the colon. -

Adaptive Tdetect Fact Sheet for Recipient
FACT SHEET FOR RECIPIENTS Coronavirus Adaptive Biotechnologies Corporation September 2, 2021 Disease 2019 T-Detect COVID Test (COVID-19) You are being given this Fact Sheet because your coughing, difficulty breathing, etc.). A full list of sample is being tested or was tested for an adaptive T- symptoms of COVID-19 can be found at the following cell immune response to the virus that causes link: https://www.cdc.gov/coronavirus/2019- Coronavirus Disease 2019 (COVID-19) using the T- ncov/symptoms-testing/symptoms.html. Detect COVID Test. How are people tested for COVID-19? Two main kinds of tests are currently available for You should not interpret the results of this COVID-19: diagnostic tests and adaptive immune response tests. test to indicate the presence or absence of immunity or protection from COVID-19 • A diagnostic test tells you if you have a current infection. infection. • An adaptive immune response test tells you if you may have had a previous infection This Fact Sheet contains information to help you understand the risks and benefits of using this test to What is the T-Detect COVID Test? evaluate your adaptive immune response to SARS-CoV- This test is similar to an antibody test in that it measures 2, the virus that causes COVID-19. After reading this your adaptive immune response to SARS-CoV-2, the Fact Sheet, if you have questions or would like to virus that causes COVID-19. However in this case it discuss the information provided, please talk to your specifically measures your adaptive T-cell immune healthcare provider. -

Reference Ranges for Blood Concentrations of Eosinophils And
Journal of Perinatology (2010) 30, 540–545 r 2010 Nature America, Inc. All rights reserved. 0743-8346/10 www.nature.com/jp ORIGINAL ARTICLE Reference ranges for blood concentrations of eosinophils and monocytes during the neonatal period defined from over 63 000 records in a multihospital health-care system RD Christensen1,2, J Jensen1,3, A Maheshwari4 and E Henry1,3 1Intermountain Healthcare Women and Newborns Clinical Program, Ogden, UT, USA; 2McKay-Dee Hospital Center, Ogden, UT, USA; 3Institute for Healthcare Delivery Research, Salt Lake City, UT, USA and 4Divisions of Neonatology and Pediatric Gastroenterology, Departments of Pediatrics, Cell Biology, and Pathology, University of Alabama at Birmingham, Birmingham, AL, USA Introduction Objective: Blood concentrations of eosinophils and monocytes are part Normal values for hematological parameters are not generally of the complete blood count. Reference ranges for these concentrations during available for neonates because blood is not drawn on healthy the neonatal period, established by very large sample sizes and modern neonates to establish normal ranges. Instead, ‘reference ranges’ are methods, are needed for identifying abnormally low or high values. used in neonatal hematology.1–6 These consist of the 5th to 95th Study Design: We constructed reference ranges for eosinophils per ml percentile values assembled from large numbers of neonates with and monocytes per ml among neonates of 22 to 42 weeks of gestation, minimal pathology or with pathology not thought to be relevant to on the day of birth, and also during 28 days after birth. Data were the laboratory parameter under study. Recent examples of their obtained from archived electronic records over an eight and one-half-year usefulness include the following: Reference ranges for erythrocyte period in a multihospital health-care system. -

Our Immune System (Children's Book)
OurOur ImmuneImmune SystemSystem A story for children with primary immunodeficiency diseases Written by IMMUNE DEFICIENCY Sara LeBien FOUNDATION A note from the author The purpose of this book is to help young children who are immune deficient to better understand their immune system. What is a “B-cell,” a “T-cell,” an “immunoglobulin” or “IgG”? They hear doctors use these words, but what do they mean? With cheerful illustrations, Our Immune System explains how a normal immune system works and what treatments may be necessary when the system is deficient. In this second edition, a description of a new treatment has been included. I hope this book will enable these children and their families to explore together the immune system, and that it will help alleviate any confusion or fears they may have. Sara LeBien This book contains general medical information which cannot be applied safely to any individual case. Medical knowledge and practice can change rapidly. Therefore, this book should not be used as a substitute for professional medical advice. SECOND EDITION COPYRIGHT 1990, 2007 IMMUNE DEFICIENCY FOUNDATION Copyright 2007 by Immune Deficiency Foundation, USA. Readers may redistribute this article to other individuals for non-commercial use, provided that the text, html codes, and this notice remain intact and unaltered in any way. Our Immune System may not be resold, reprinted or redistributed for compensation of any kind without prior written permission from Immune Deficiency Foundation. If you have any questions about permission, please contact: Immune Deficiency Foundation, 40 West Chesapeake Avenue, Suite 308, Towson, MD 21204, USA; or by telephone at 1-800-296-4433. -

Defining Natural Antibodies
PERSPECTIVE published: 26 July 2017 doi: 10.3389/fimmu.2017.00872 Defining Natural Antibodies Nichol E. Holodick1*, Nely Rodríguez-Zhurbenko2 and Ana María Hernández2* 1 Department of Biomedical Sciences, Center for Immunobiology, Western Michigan University Homer Stryker M.D. School of Medicine, Kalamazoo, MI, United States, 2 Natural Antibodies Group, Tumor Immunology Division, Center of Molecular Immunology, Havana, Cuba The traditional definition of natural antibodies (NAbs) states that these antibodies are present prior to the body encountering cognate antigen, providing a first line of defense against infection thereby, allowing time for a specific antibody response to be mounted. The literature has a seemingly common definition of NAbs; however, as our knowledge of antibodies and B cells is refined, re-evaluation of the common definition of NAbs may be required. Defining NAbs becomes important as the function of NAb production is used to define B cell subsets (1) and as these important molecules are shown to play numerous roles in the immune system (Figure 1). Herein, we aim to briefly summarize our current knowledge of NAbs in the context of initiating a discussion within the field of how such an important and multifaceted group of molecules should be defined. Edited by: Keywords: natural antibody, antibodies, natural antibody repertoire, B-1 cells, B cell subsets, B cells Harry W. Schroeder, University of Alabama at Birmingham, United States NATURAL ANTIBODY (NAb) PRODUCING CELLS Reviewed by: Andre M. Vale, Both murine and human NAbs have been discussed in detail since the late 1960s (2, 3); however, Federal University of Rio cells producing NAbs were not identified until 1983 in the murine system (4, 5). -

Adaptive Immune Systems
Immunology 101 (for the Non-Immunologist) Abhinav Deol, MD Assistant Professor of Oncology Wayne State University/ Karmanos Cancer Institute, Detroit MI Presentation originally prepared and presented by Stephen Shiao MD, PhD Department of Radiation Oncology Cedars-Sinai Medical Center Disclosures Bristol-Myers Squibb – Contracted Research What is the immune system? A network of proteins, cells, tissues and organs all coordinated for one purpose: to defend one organism from another It is an infinitely adaptable system to combat the complex and endless variety of pathogens it must address Outline Structure of the immune system Anatomy of an immune response Role of the immune system in disease: infection, cancer and autoimmunity Organs of the Immune System Major organs of the immune system 1. Bone marrow – production of immune cells 2. Thymus – education of immune cells 3. Lymph Nodes – where an immune response is produced 4. Spleen – dual role for immune responses (especially antibody production) and cell recycling Origins of the Immune System B-Cell B-Cell Self-Renewing Common Progenitor Natural Killer Lymphoid Cell Progenitor Thymic T-Cell Selection Hematopoetic T-Cell Stem Cell Progenitor Dendritic Cell Myeloid Progenitor Granulocyte/M Macrophage onocyte Progenitor The Immune Response: The Art of War “Know your enemy and know yourself and you can fight a hundred battles without disaster.” -Sun Tzu, The Art of War Immunity: Two Systems and Their Key Players Adaptive Immunity Innate Immunity Dendritic cells (DC) B cells Phagocytes (Macrophages, Neutrophils) Natural Killer (NK) Cells T cells Dendritic Cells: “Commanders-in-Chief” • Function: Serve as the gateway between the innate and adaptive immune systems. -

The RAG Recombinase Dictates Functional Heterogeneity and Cellular Fitness in Natural Killer Cells
The RAG Recombinase Dictates Functional Heterogeneity and Cellular Fitness in Natural Killer Cells Jenny M. Karo,1 David G. Schatz,2 and Joseph C. Sun1,* 1Immunology Program and Gerstner Sloan Kettering Graduate School of Biomedical Sciences, Memorial Sloan Kettering Cancer Center, New York, NY 10065, USA 2Department of Immunobiology and the Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT 06510, USA *Correspondence: [email protected] http://dx.doi.org/10.1016/j.cell.2014.08.026 SUMMARY completely absent (Mombaerts et al., 1992; Shinkai et al., 1992). There is as yet no evidence that RAG plays a physiological The emergence of recombination-activating genes role in any cell type other than B and T lymphocytes or in any pro- (RAGs) in jawed vertebrates endowed adaptive cess other than V(D)J recombination. immune cells with the ability to assemble a diverse Although NK cells have long been classified as a component set of antigen receptor genes. In contrast, innate of the innate immune system, recent evidence suggests that lymphocytes, such as natural killer (NK) cells, are this cell type possesses traits attributable to adaptive immunity not believed to require RAGs. Here, we report that (Sun and Lanier, 2011). These characteristics include ‘‘educa- tion’’ mechanisms to ensure self-tolerance during NK cell devel- NK cells unable to express RAGs or RAG endonu- opment and clonal-like expansion of antigen-specific NK clease activity during ontogeny exhibit a cell-intrinsic cells during viral infection, followed by the ability to generate hyperresponsiveness but a diminished capacity long-lived ‘‘memory’’ NK cells. -
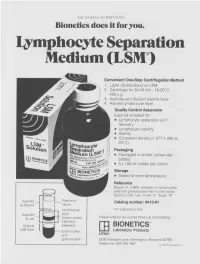
Lymphocyte Separation Medium (LSM
THE JOURNAL OF IMMUNOLOGY Bionetics does it for you. Lymphocyte Separation Medium (LSM wenient One-Step Centrifugation Method _ayer diluted blood on LSM. 2,entrifuge for 30-40 min., 18-20°C, ~.00 x g. ~,spirate and discard plasma layer -larvest lymphocyte layer. Quality Control Assurance Each lot is tested for: • Lymphocyte separation and recovery. • Lymphocyte viability. • Sterility. • Consistent density (1.077-1.080 at 20°C). Packaging • Packaged in amber, screw-cap bottles. • 5 x 100 ml bottles per carton. Storage. • Stored at room temperature. Reference Boyum, A. (1968): Isolation of mononuclear cells and granulocytes from human blood. Scand J. Clin. Lab. Invest. 21, Suppl. 97. Aspirate I IC[OI I IC~ LJI Catalog number: 8410-01 & discard serum Lymphocyte For Laboratory Use Aspirate layer & use (mononuclear Please write for our current Price List and Catalog. cells and Original platelets) ITi BIONETICS° LSM layer Erythrocytes Laboratory Products and Litton granulocytes 5516 Nicholson Lane, Kensington, Maryland 20795 Telephone: (301) 881-1557 1979 Litton Bionetics, tnc Get the most out of your high quality cytotoxic antibodies with LOW-TOX-M RABBIT COMPLEMENT LOW TOXICITY HIGH ACTIVITY Presentation: CL 3051 5 x 1 ml, lyophilized $30.00 When it comes to COMPLEMENT... come to CEDARLANE Direct orders or inquiries to: UNITED STATES: WORLDWIDE EXCEPT U.S. ,4 C~L CEDARLANE ACCURATE CHEMICAL & LABORATO RI ES SCIENTIFIC CORPORATION LIMITED 5516-8TH LINE, R.R. 2 28 TEC STREET, HICKSVtLLE, N.Y. 11801 HORNBY, ONTARIO, CANADA LOP 1E0 Telephone