Chemical Properties of Potassium Ferrocyanide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Problem 11 the Prussian Blue the Prussian Blue Is a Component Of
www.pianetachimica.it Problem 11 The Prussian blue The Prussian blue is a component of black and bluish inks for printing. The precipitate formed upon the dropwise addition of potassium ferrocyanide to the ferric chloride solution contains 34.9% (mass) of iron. 1. Give a formulae of the precipitate and write the chemical equation. 2. Using the crystal field theory show the d-orbital splitting pattern for all Fe atoms in the Prussian blue. 3. What causes the intense color of the pigment? 4. What product initially forms from potassium ferrocyanide and ferric chloride solution in the inverse- mixing-order route? Give the equation. Solution. 1. Let's write the chemical equation : 4 FeCl3 + 3 K4[Fe(CN)6] ® Fe4[Fe(CN)6]3 + 12 KCl where Fe4[Fe(CN)6]3 is the Prussian blue or Berlin blue. Problem is that adding drops of ferrocyanide to ferric chloride it could appear the precipitate of the form Fe4[Fe(CN)6]3 . aH2O (a = 14 up to 16) or even Fe4[Fe(CN)6-xClx]3 .a H2O. In the first case molecular mass is 859.23 +18a and contains 390.95 g Fe. So, a = (39095 - 859.23x34.9)/(18x34.9) = 14.5. Not acceptable because a is not integer. For the second case we have molar mass 859.23 +35.5x - 26x + 18a containing 390.95 g Fe. So, we have 39095- 859.23x34.9 = 34.9 x 9 x (X +2a) where x +2a = 29. Convenient are x =1 and a =14 or x = 3 and a = 13. -

Excess Heat Production in the Redox Couple Reaction of Ferricyanide and Ferrocyanide
www.nature.com/scientificreports OPEN Excess heat production in the redox couple reaction of ferricyanide and ferrocyanide Atsushi Sugiyama1,2,3*, Makoto Miura4, Yoshinobu Oshikiri5, Yena Kim3, Ryoichi Morimoto6, Miki Miura7, Tetsuya Osaka2, Iwao Mogi8, Yusuke Yamauchi3,9* & Ryoichi Aogaki3,10* In order to establish the universality of the excess heat production in electrochemical reaction, under a high magnetic feld, as one of the most fundamental electrochemical reactions, the case of ferricyanide-ferrocyanide redox reaction was examined, where ionic vacancies with ± 1 unit charge were collided by means of magnetohydrodynamic (MHD) fow. As a result, from the pair annihilation of the vacancies with opposite signs, beyond 7 T, excess heat production up to 25 kJ·mol−1 in average at 15 T was observed, which was attributed to the liberation of the solvation energy stored in a pair of the vacancy cores with a 0.32 nm radius, i.e., 112 kJ·mol−1. Diference between the observed and expected energies comes from the small collision efciency of 0.22 due to small radius of the vacancy core. Ionic vacancy initially created as a by-product of electrode reaction is unstable in solution phase, stabilized by releasing solvation energy. Ionic vacancy utilizes the energy to enlarge the core and stores the energy in it. As a result, solvated ionic vacancy consists of a polarized free space of the enlarged core surrounded by oppositely charged ionic cloud. The accuracy and precision of the measured values were ascertained by in situ standard additive method. Recently, in copper redox reaction under a high magnetic feld, great excess heat production up to 410 kJ·mol−1 in average has been observed, which is 1.5 times larger than the molar combustion heat of hydrogen (285.8 kJ·mol−1)1. -

Preparation of 15N-Labeled Potassium Ferrocyanide for Tracer Studies
Environment and Pollution; Vol. 6, No. 2; 2017 ISSN 1927-0909 E-ISSN 1927-0917 Published by Canadian Center of Science and Education Preparation of 15N-labeled Potassium Ferrocyanide for Tracer Studies Tsvetelina Dimitrova1, Frank Repmann1 & Dirk Freese1 1 Chair of Soil Protection and Recultivation, Brandenburg University of Technology Cottbus-Senftenberg, Konrad-Wachsmann-Allee 6, D-03046 Cottbus, Germany Correspondence: Tsvetelina Dimitrova, Brandenburg University of Technology Cottbus-Senftenberg, Konrad-Wachsmann-Allee 6, D-03046 Cottbus, Germany. Tel: 49-355-69-4337. E-mail: [email protected] Received: August 4, 2017 Accepted: August 22, 2017 Online Published: September 29, 2017 doi:10.5539/ep.v6n2p41 URL: https://doi.org/10.5539/ep.v6n2p41 Abstract Isotopic labels are widely used to trace the fate and cycling of common environmental contaminants. Many of the labeled materials are not available commercially and, depending on the complexity of the substance, the label and the enrichment level, custom syntheses are costly. A simple, straightforward, and cost effective method for 15 15 the preparation of a highly enriched, N-labeled potassium ferrocyanide (K4[Fe(C N)6]*3H2O) has been developed to meet the requirements of related tracer experiments and minimize their costs. In this case, the 15N label was used to quantify iron cyanide detoxification (biodegradation and/or transformation) within soil-plant-systems. 15N-labeled potassium cyanide (KC15N) and a ferrous iron salt have been used for the synthesis. Extensive qualitative and quantitative analyses showed a product, entirely identical in its functional 15 and elemental components to commercial non-labeled K4[Fe(CN)6]*3H2O and in its N enrichment to the KC15N used for its synthesis. -

Reversibility of Ferri-/Ferrocyanide Redox During Operando Soft X-Ray Spectroscopy
Reversibility of Ferri-/Ferrocyanide Redox during Operando Soft X-ray Spectroscopy The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Risch, Marcel, Kelsey A. Stoerzinger, Tom Z. Regier, Derek Peak, Sayed Youssef Sayed, and Yang Shao-Horn. “Reversibility of Ferri-/ Ferrocyanide Redox During Operando Soft X-Ray Spectroscopy.” The Journal of Physical Chemistry C 119, no. 33 (August 20, 2015): 18903–18910. As Published http://dx.doi.org/10.1021/acs.jpcc.5b04609 Publisher American Chemical Society (ACS) Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/109590 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Reversibility of Ferri-Ferrocyanide Redox During Operando Soft X-Ray Spectroscopy ┴ Marcel Risch,†* Kelsey A. Stoerzinger,§ Tom Z. Regier,‡ Derek Peak,º Sayed Youssef Sayed,† Yang Shao-Horn†§||* †Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, MA, USA 02139 §Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA 02139 ||Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA 02139 ‡Canadian Light Source, Inc., Saskatoon, SK, Canada S7N 2V3 ºDepartment of Soil Sciences, University of Saskatchewan, Saskatoon, SK, Canada S7N 5A8 KEYWORDS. Electrochemistry; In-situ; redox shuttle; iron cyanide; X-ray absorption; radiolysis; radiation damage. ABSTRACT The ferri-ferrocyanide redox couple is ubiquitous in many fields of physical chemistry. We studied its photochemical response to intense synchrotron radiation by in-situ X-Ray absorption spectroscopy. -
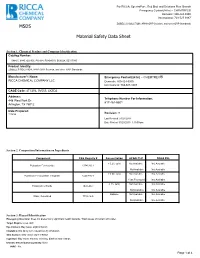
MSDS Material Safety Data Sheet
For RICCA, SpectroPure, Red Bird, and Solutions Plus Brands Emergency Contact(24 hr) -- CHEMTREC® Domestic: 800-424-9300 International: 703-527-3887 ZOBELL'S SOLUTION, APHA ORP Solution, and other ORP Standards MSDS Material Safety Data Sheet Section 1: Chemical Product and Company Identification Catalog Number: 5464.5, 9880, OX-905, PX-918, R5464510, S0932A, SZ117300 Product Identity: ZOBELL'S SOLUTION, APHA ORP Solution, and other ORP Standards Manufacturer's Name: Emergency Contact(24 hr) -- CHEMTREC® RICCA CHEMICAL COMPANY LLC Domestic: 800-424-9300 International: 703-527-3887 CAGE Code: 4TCW6, 0V553, 4XZQ2 Address: Telephone Number For Information: 448 West Fork Dr 817-461-5601 Arlington, TX 76012 Date Prepared: 11/9/98 Revision: 9 Last Revised: 01/24/2014 Date Printed: 03/25/2015 1:19:09 pm Section 2. Composition/Information on Ingredients Component CAS Registry # Concentration ACGIH TLV OSHA PEL < 0.2% (w/v) Not Available Not Available Potassium Ferricyanide 13746-66-2 Not Available Not Available < 0.5% (w/v) Not Available Not Available Potassium Ferrocyanide Trihydrate 14459-95-1 1 (as Fe) mg/m3 Not Available < 1% (w/v) Not Available Not Available Potassium Chloride 7447-40-7 Not Available Not Available Balance Not Available Not Available Water, Deionized 7732-18-5 Not Available Not Available Section 3: Hazard Identification Emergency Overview: Does not present any significant health hazards. Wash areas of contact with water. Target Organs: eyes, skin Eye Contact: May cause slight irritation. Inhalation: Not likely to be hazardous by inhalation. Skin Contact: May cause slight irritation. Ingestion: May cause nausea, vomiting, diarrhea and cramps. -

No, Potassium Ferrocyanide in Some Salt Brands Is Not at Toxic Levels
Fact check: No, potassium ferrocyanide in some salt brands is not at toxic levels Shubashree Desikan JULY 02, 2019 Citing a report from U.S.-based West American Analytical Laboratories, Shiv Shankar Gupta, chairman of Godhum Grains and Food Products, accused some salt manufacturers in India of selling food-grade salt that contained high levels of potassium ferrocyanide. This caused a panic, which was aggravated by widespread sharing of the news item on social media. Indeed, potassium ferrocyanide is being used in salt to give it anti-caking properties. But the answer to whether it is toxic is no! Firstly, while potassium cyanide is a toxic substance and releases the cyanide anion when consumed by a person, potassium ferrocyanide is not. In potassium ferrocyanide, the cyanide anion is strongly bonded to ferrous ion and hence does not get hydrolysed. An image widely shared in social media. Secondly, there is the factor known as the LD50 value. This is expanded as “Lethal Dose 50%”. LD50 is the amount of any chemical that can cause death in 50% of the group of animals that it enters by consumption or absorption through the skin. According to inorganic chemist Dr. Sayam Sengupta of IISER Kolkata, the LD50 value for common salt — sodium chloride — for rats is greater than 3 gram per kilogram body weight of the animal. He points out that for potassium Ferrocyanide, LD 50 value is 3.6 gram per kilogram weight of the animal, when taken orally. As this indicates, the LD50 value of potassium ferrocyanide is almost the same as common salt. -

On the Cyanogen Halides by P
ON THE CYANOGEN HALIDES BY P. KAILASAM (From the Chemistry Department, Madras Christian College) Received July 31, 1941 (Communicated by Sir C. V. Raman, xt., F.R.S., NN.L.) I. Action of Halogens on Cyanogen Halides No observations appear to have been recorded on the action of halogens on cyanogen halides except that of chlorine on cyanogen chloride (Wurtz, 1851; Naumann and Vogt, 1870) and bromine on cyanogen bromide in ether (Poonamarew, 1885) which polymerise them to cyanuric chloride and cyanuric bromide respectively. The object of the present investigation is to study the possible displacement reactions with the cyanogen halides. The action of chlorine gas on cyanogen iodide and the action of bromine on cyanogen chloride were first studied. It was expected that chlorine would displace iodine from cyanogen iodide forming cyanogen chloride, but it was found that cyanogen iodide was not affected by dry chlorine. Dry cyanogen iodide, prepared by warming an intimate mixture of one part of iodine and two parts of AgCN, at about 40°, was sublimed into a narrow tube which could be heated in a water-bath. Dry chlorine was passed through the tube for nearly two hours. No immediate reaction occurred, and even after leaving for a week at room temperature (30°) the white needles of cyanogen iodide remained unchanged. The tube was then slowly heated, but no reaction occurred up to the sublimation temperature of cyanogen iodide (about 40°). It was found that cyanogen iodide could be sublimed unchanged in an atmosphere of dry chlorine. Since chlorine does not displace iodine from cyanogen iodide, it was thought that bromine might displace chlorine from cyanogen chloride form- ing cyanogen bromide. -

The Light Triggered Dissolution of Gold Wires Using Potassium Ferrocyanide T Solutions Enables Cumulative Illumination Sensing ⁎ Weida D
Sensors & Actuators: B. Chemical 282 (2019) 52–59 Contents lists available at ScienceDirect Sensors and Actuators B: Chemical journal homepage: www.elsevier.com/locate/snb The light triggered dissolution of gold wires using potassium ferrocyanide T solutions enables cumulative illumination sensing ⁎ Weida D. Chena, Seung-Kyun Kangb, Wendelin J. Starka, John A. Rogersc,d,e, Robert N. Grassa, a Department of Chemistry and Applied Biosciences, ETH Zurich, Vladimir-Prelog-Weg 1, 8093 Zurich, Switzerland b Department of Bio and Brain Engineering, KAIST, 291 Daehak-ro, Yuseong-gu, Daejeon 334141, Republic of Korea c Departments of Materials Science and Engineering, Biomedical Engineering, Neurological Surgery, Chemistry, Mechanical Engineering, Electrical Engineering and Computer Science, Northwestern University, Evanston, IL 60208, USA d Center for Bio-Integrated Electronics, Northwestern University, Evanston, IL 60208, USA e Simpson Querrey Institute for Nano/Biotechnology, Northwestern University, Evanston, IL 60208, USA ARTICLE INFO ABSTRACT Keywords: Electronic systems with on-demand dissolution or destruction capabilities offer unusual opportunities in hard- Photochemistry ware-oriented security devices, advanced military spying and controlled biological treatment. Here, the dis- Cyanide solution chemistry of gold, generally known as inert metal, in potassium ferricyanide and potassium ferrocya- Sensor nide solutions has been investigated upon light exposure. While a pure aqueous solution of potassium Conductors ferricyanide–K3[Fe(CN)6] does not dissolve gold, an aqueous solution of potassium ferrocyanide–K4[Fe(CN)6] Diffusion limitation irradiated with ambient light is able to completely dissolve a gold electrode within several minutes. Photo Devices activation and dissolution kinetics were assessed at different initial pH values, light irradiation intensities and ferrocyanide concentrations. -

Potassium Ferrocyanide Wine Treatment: a Controversial, Yet Necessary Operation
Scien. Tech. Bull-Chem. Food Sci. Eng., Vol. 14(XV), 2017, 4-8 POTASSIUM FERROCYANIDE WINE TREATMENT: A CONTROVERSIAL, YET NECESSARY OPERATION Ionel POPESCU-MITROI,* Dana RADU Faculty of Food Engineering, Tourism and Environmental Protection, “Aurel Vlaicu” University, Romania, 2 Elena Dragoi St., Arad 310330, Romania Corresponding author email: [email protected] Abstract: This paper presents the method of iron removal from wine by using a potassium ferrocyanide treatment, a method implemented in Romania by oenologist Ştefan Teodorescu, which became official in 1973. Potassium ferrocyanide treatment is a controversial operation that poses safety and environmental protection problems, but is absolutely necessary for the efficient removal of iron from wines rich in this cation. Without iron removal, wines with over 10 mg/l of iron become susceptible to fearsome chemical defects, such as turning whiter, darker or bluisher. The paper presents a case study for the white wine Fetească Regală, obtained in the Recaş vineyard, harvested in 2016, in order to establish the theoretical and practical doses of potassium ferrocyanide based on microprobes, to remove the iron up to the safety threshold (4 mg Fe total/l of wine). The conclusions of the paper include some “good practices” for the success of the treatment and the prevention of toxicity problems. Keywords: potassium ferrocyanide, wine, microprobes, iron removal. INTRODUCTION and bluisher in color, which occurs both in By mineral nutrition, the wine accrues small white and red wines (Gheorghiţă et al., 2002). amounts of iron in grapes: 2-3 mg/kg. In In order to prevent the occurrence of these addition to the mineral substances derived from chemical defects and for a better stabilization grapes, wine is enriched with significant of the wine, it is necessary to remove the iron amounts of exogenous mineral substances from the wine. -

Safety Data Sheet
Safety Data Sheet Classified According to OSHA Hazard Communication Standard (HCS) SECTION 1: Identification 1.1. Product Identifier Trade Name or Designation: ORP Standard, +200 mV vs. Ag/AgCl (4M KCl sat. with AgCl filling solution) Product Number: 5464.5 Other Identifying Product Numbers: 5464.5-1, 5464.5-16, 5464.5-2.5, 5464.5-32, 5464.5-4, 5464.5-5 1.2. Recommended Use and Restrictions on Use General Laboratory Reagent 1.3. Details of the Supplier of the Safety Data Sheet Company: Ricca Chemical Company Address: 448 West Fork Drive Arlington, TX 76012 USA Telephone: 888-467-4222 1.4. Emergency Telephone Number (24 hours) CHEMTREC (USA) 800-424-9300 CHEMTREC (International) 1+ 703-527-3887 SECTION 2: Hazard(s) Identification 2.1. Classification of the Substance or Mixture For the full text of the Hazard and Precautionary Statements listed below, see Section 16. This Product is not categorized as hazardous in any GHS hazard class. 2.2. GHS Label Elements Pictograms: None Required. Signal Word: None Required. Product Number: 5464.5 Page 1 of 10 Safety Data Sheet Hazard Statements: None Required. Precautionary Statements: None Required. 2.4. Hazards not Otherwise Classified or Covered by GHS Data not available. SECTION 3: Composition / Information on Ingredients 3.1. Components of Substance or Mixture Chemical Name Formula Molecular Weight CAS Number Weight% Water H₂O 18.01 g/mol 7732-18-5 98.92 Potassium Chloride KCl 74.55 g/mol 7447-40-7 0.75 Potassium Ferrocyanide Trihydrate K₄Fe(CN)₆·3H₂O 422.39 g/mol 14459-95-1 0.30 Potassium Ferricyanide K₃Fe(CN)₆ 329.24 g/mol 13746-66-2 0.03 SECTION 4: First-Aid Measures 4.1. -

Potassium Ferrocyanide, 2%
Potassium Ferrocyanide, 2% 1. Identification Product Name: Potassium Ferrocyanide, 2% Item #: MA0103028, MA0103053, SKC1052-250, SKC1052-500 Web SDS: S169 Synonyms: Potassium Hexacyanoferrate (II) Trihydrate Solution Recommended Use: N/A Restrictions on Use: N/A Manufacturer: In Case of Emergency: BBC Biochemical Chemtrec US 1-800-424-9300 409 Eleanor Lane, Chemtrec International 703-527-3887 Mount Vernon, WA 98273 1-800-635-4477 2. Hazards Identification OSHA Hazard Classification(s): No OSHA Hazard Classifications Applicable Signal Word: N/A Hazard Statement(s): N/A Pictogram(s): N/A Precautionary Statement(s): None applicable. Descriptions of Hazards not otherwise classified: N/A Percent of mixture with unknown acute toxicity: N/A 3. Composition and Information on Ingredients Chemical Name Common Name CAS # Concentration % Water 7732-18-5 98 Potassium Ferrocyanide 14459-95-1 2 Trihdyrate 4. First Aid Measures Eye Contact: Flush eyes with water as a precaution. Skin Contact: Wash off with plenty of water. Remove contaminated clothing and launder before reuse as a precaution. Inhalation: Move person to fresh air; give artificial respiration if breathing has stopped. Ingestion: Never give anything by mouth to an unconscious person. Rinse mouth with water. Get medical attention if discomfort occurs. Symptoms: N/A Recommendations for immediate medical care/special treatment: If exposure by any route causes irritation get medical advice/attention. 5. Fire- Fighting Measures Extinguishing Media: Dry chemical, carbon dioxide, alcohol foam, water. Fire Hazards (Chemical): Not flammable. Special Protective Equipment: Fire fighters should use self-contained breathing apparatus and protective clothing. Precautions for Firefighters: Fire fighters should use self-contained breathing apparatus and protective clothing. -

Potassium Ferrocyanide Trihydrate
SAFETY DATA SHEET Creation Date 10-Feb-2011 Revision Date 19-Jan-2018 Revision Number 3 1. Identification Product Name Potassium ferrocyanide trihydrate Cat No. : AC211090000; AC211090025; AC211095000 CAS-No 14459-95-1 Synonyms Potassium hexacyanoferrate(II) Recommended Use Laboratory chemicals. Uses advised against Food, drug, pesticide or biocidal product use. Details of the supplier of the safety data sheet Company Fisher Scientific Acros Organics One Reagent Lane One Reagent Lane Fair Lawn, NJ 07410 Fair Lawn, NJ 07410 Tel: (201) 796-7100 Emergency Telephone Number For information US call: 001-800-ACROS-01 / Europe call: +32 14 57 52 11 Emergency Number US:001-201-796-7100 / Europe: +32 14 57 52 99 CHEMTREC Tel. No.US:001-800-424-9300 / Europe:001-703-527-3887 2. Hazard(s) identification Classification Classification under 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Label Elements None required Hazards not otherwise classified (HNOC) Harmful to aquatic life with long lasting effects WARNING. Cancer and Reproductive Harm - https://www.p65warnings.ca.gov/. 3. Composition/Information on Ingredients ______________________________________________________________________________________________ Page 1 / 7 Potassium ferrocyanide trihydrate Revision Date 19-Jan-2018 ______________________________________________________________________________________________ Component CAS-No Weight % Potassium ferrocyanide trihydrate 14459-95-1 >95 Ferrate(4-), hexakis(cyano-C)-, tetrapotassium, 13943-58-3 - (OC-6-11)- 4. First-aid measures Eye Contact Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. Get medical attention. Skin Contact Wash off immediately with plenty of water for at least 15 minutes. Get medical attention immediately if symptoms occur. Inhalation Remove to fresh air.