Evaluating Breeding Performance in Mixed Species Bird Enclosures Within European Zoos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
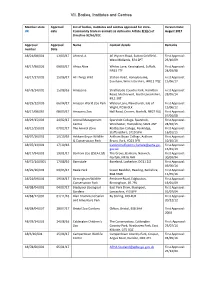
VII. Bodies, Institutes and Centres
VII. Bodies, Institutes and Centres Member state Approval List of bodies, institutes and centres approved for intra- Version Date: UK date Community trade in animals as defined in Article 2(1)(c) of August 2017 Directive 92/65/EEC Approval Approval Name Contact details Remarks number Date AB/21/08/001 13/03/17 Ahmed, A 46 Wyvern Road, Sutton Coldfield, First Approval: West Midlands, B74 2PT 23/10/09 AB/17/98/026 09/03/17 Africa Alive Whites Lane, Kessingland, Suffolk, First Approval: NR33 7TF 24/03/98 AB/17/17/005 15/06/17 All Things Wild Station Road, Honeybourne, First Approval: Evesham, Worcestershire, WR11 7QZ 15/06/17 AB/78/14/002 15/08/16 Amazonia Strathclyde Country Park, Hamilton First Approval: Road, Motherwell, North Lanarkshire, 28/05/14 ML1 3RT AB/29/12/003 06/04/17 Amazon World Zoo Park Watery Lane, Newchurch, Isle of First Approval: Wight, PO36 0LX 15/06/12 AB/17/08/065 08/03/17 Amazona Zoo Hall Road, Cromer, Norfolk, NR27 9JG First Approval: 07/04/08 AB/29/15/003 24/02/17 Animal Management Sparsholt College, Sparsholt, First Approval: Centre Winchester, Hampshire, SO21 2NF 24/02/15 AB/12/15/001 07/02/17 The Animal Zone Rodbaston College, Penkridge, First Approval: Staffordshire, ST19 5PH 16/01/15 AB/07/16/001 10/10/16 Askham Bryan Wildlife Askham Bryan College, Askham First Approval: & Conservation Park Bryan, York, YO23 3FR 10/10/16 AB/07/13/001 17/10/16 [email protected]. First Approval: gov.uk 15/01/13 AB/17/94/001 19/01/17 Banham Zoo (ZSEA Ltd) The Grove, Banham, Norwich, First Approval: Norfolk, NR16 -
Delivering a Sustainable International Visitors
Whitsun Campaign Delivering a Sustainable International Visitor Attraction…….a long road, with many successes and the odd bump! Creating Sustainable Tourism Destinations Ray Morrison, University of Chester Facilities & Environment Manager 9th July 2012 Chester Zoo Chester Zoo.......... Who are we? Our mission? Do we deliver? Do we operate the business sustainably? Key sustainability issues? Key achievements? Questions and Answers (maybe) Coffee and (maybe?) Who are we? Formed in 1934 Registered charity, conservation and education UK’s No. 1 Wildlife Attraction Top 15 zoos in World (Forbes Magazine) 1.4 million visitors in 2011 8,000 Animals, 400 different species 1,000 of Plants many endangered or rare Turnover £28 million per annum £1 million invested annually in conservation projects worldwide • Vision and Mission? Our Vision - A diverse, thriving and sustainable natural world Our Mission - To be a major force in conserving biodiversity worldwide Strategic Objective - To manage our work and activities to ensure long-term sustainability. Diverse and complex business Insight into our animal, plant, educational and environmental activities Do we deliver? ‘Sustainable’ …. Satisfying the needs of today without compromising tomorrow • Do we deliver on our mission? Evidence – Sustained achievements in line with our animal and plant conservation and educational goals, local and national. • Do we manage the Zoo’s operations sustainably? Evidence – ISO 14001, continual improvement, achieved various local and national awards Delivering -

Verzeichnis Der Europäischen Zoos Arten-, Natur- Und Tierschutzorganisationen
uantum Q Verzeichnis 2021 Verzeichnis der europäischen Zoos Arten-, Natur- und Tierschutzorganisationen Directory of European zoos and conservation orientated organisations ISBN: 978-3-86523-283-0 in Zusammenarbeit mit: Verband der Zoologischen Gärten e.V. Deutsche Tierpark-Gesellschaft e.V. Deutscher Wildgehege-Verband e.V. zooschweiz zoosuisse Schüling Verlag Falkenhorst 2 – 48155 Münster – Germany [email protected] www.tiergarten.com/quantum 1 DAN-INJECT Smith GmbH Special Vet. Instruments · Spezial Vet. Geräte Celler Str. 2 · 29664 Walsrode Telefon: 05161 4813192 Telefax: 05161 74574 E-Mail: [email protected] Website: www.daninject-smith.de Verkauf, Beratung und Service für Ferninjektionsgeräte und Zubehör & I N T E R Z O O Service + Logistik GmbH Tranquilizing Equipment Zootiertransporte (Straße, Luft und See), KistenbauBeratung, entsprechend Verkauf undden Service internationalen für Ferninjektionsgeräte und Zubehör Vorschriften, Unterstützung bei der Beschaffung der erforderlichenZootiertransporte Dokumente, (Straße, Vermittlung Luft und von See), Tieren Kistenbau entsprechend den internationalen Vorschriften, Unterstützung bei der Beschaffung der Celler Str.erforderlichen 2, 29664 Walsrode Dokumente, Vermittlung von Tieren Tel.: 05161 – 4813192 Fax: 05161 74574 E-Mail: [email protected] Str. 2, 29664 Walsrode www.interzoo.deTel.: 05161 – 4813192 Fax: 05161 – 74574 2 e-mail: [email protected] & [email protected] http://www.interzoo.de http://www.daninject-smith.de Vorwort Früheren Auflagen des Quantum Verzeichnis lag eine CD-Rom mit der Druckdatei im PDF-Format bei, welche sich großer Beliebtheit erfreute. Nicht zuletzt aus ökologischen Gründen verzichten wir zukünftig auf eine CD-Rom. Stattdessen kann das Quantum Verzeichnis in digitaler Form über unseren Webshop (www.buchkurier.de) kostenlos heruntergeladen werden. Die Datei darf gerne kopiert und weitergegeben werden. -

Tour Report 1 – 8 January 2016
The Gambia - In Style! Naturetrek Tour Report 1 – 8 January 2016 White-throated Bee-eaters Violet Turaco by Kim Taylor African Wattled Lapwing Blue-bellied Roller Report compiled by Marcus John Images courtesy of Kim Taylor & Marcus John Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk The Gambia - In Style! Tour Report Tour Participants: Marcus John (leaders) with six Naturetrek clients Summary The Gambia is an ideal destination for a relaxed holiday and offers a great introduction to the diverse and colourful birdlife of Africa. We spent the week at the stunning Mandina Lodges, a unique place that lies on a secluded mangrove-lined tributary of the mighty River Gambia. The lodges are situated next to the creek and within the Makasuto Forest, which comprises over a thousand acres of pristine, protected forest. Daily walks took us out through the woodland and into the rice fields and farmland beyond, where a great range of birds and butterflies can be found. It was sometimes hard to know where to look as parrots, turacos, rollers and bee-eaters all vied for our attention! Guinea Baboons are resident in the forest and were very approachable; Green Vervet Monkeys were seen nearly every day and we also found a group of long-limbed Patas Monkeys, the fastest primates in the world! Boat trips along the creek revealed a diverse selection of waders, kingfishers and other waterbirds; fourteen species of raptor were also seen during the week. -

ATIC0943 {By Email}
Animal and Plant Health Agency T 0208 2257636 Access to Information Team F 01932 357608 Weybourne Building Ground Floor Woodham Lane www.gov.uk/apha New Haw Addlestone Surrey KT15 3NB Our Ref: ATIC0943 {By Email} 4 October 2016 Dear PROVISION OF REQUESTED INFORMATION Thank you for your request for information about zoos which we received on 26 September 2016. Your request has been handled under the Freedom of Information Act 2000. The information you requested and our response is detailed below: “Please can you provide me with a full list of the names of all Zoos in the UK. Under the classification of 'Zoos' I am including any place where a member of the public can visit or observe captive animals: zoological parks, centres or gardens; aquariums, oceanariums or aquatic attractions; wildlife centres; butterfly farms; petting farms or petting zoos. “Please also provide me the date of when each zoo has received its license under the Zoo License act 1981.” See Appendix 1 for a list that APHA hold on current licensed zoos affected by the Zoo License Act 1981 in Great Britain (England, Scotland and Wales), as at 26 September 2016 (date of request). The information relating to Northern Ireland is not held by APHA. Any potential information maybe held with the Department of Agriculture, Environment and Rural Affairs Northern Ireland (DAERA-NI). Where there are blanks on the zoo license start date that means the information you have requested is not held by APHA. Please note that the Local Authorities’ Trading Standard departments are responsible for administering and issuing zoo licensing under the Zoo Licensing Act 1981. -

A Stella Year the Park Is Celebrating Its 50Th Anniversary in 2020
2020 WILD TALKwww.cotswoldwildlifepark.co.uk New baby White Rhino Stella cuddles up to her mum Ruby Photo: Rory Carnegie Rory Photo: A Stella Year The Park is celebrating its 50th Anniversary in 2020. We hope to continue to inspire future Ruby and Stella walked out of the stall, giving generations to appreciate the beauty of the lucky visitors a glimpse of a baby White Rhino natural world. 2019 was a great year with Soon after the birth, Ruby and her new calf record visitor numbers, TV appearances, lots walked out of the stall into the sunshine of the of baby animals and a new Rhino calf. yard, giving a few lucky visitors a glimpse of a baby White Rhino less than two hours old. ighlights of the year included the Stella is doing well and Ruby has proved once Park featuring on BBC’s Springwatch again to be an exceptional mother. Having Hprogramme, with our part in the White another female calf is really important for the Storks’ UK re-introduction project. Then in European Breeding Programme of this iconic Astrid October, BBC Gardeners’ World featured the but endangered species. Park and presenter Adam Frost sang the praises OUR 6 RHINO CALVES of our gardens. To top off the year, Ruby gave White Rhinos have always been an important 1. Astrid born 1st July 2013, birth to our sixth Rhino calf in as many years, species at the Park, which was founded by John moved to Colchester Zoo. named “Stella”! Heyworth (1925-2012) in 1970. He had a soft spot 2. -

San Blas Checklist-2019
San Blas & Durango Highway Otus asio Tours COMMON NAME SCIENTIFIC NAME 3/6 3/7 3/8 3/9 3/10 3/11 3/12 3/13 3/14 3/15 3/16 ANATIDAE 1. Black-bellied Whistling-Duck Dendrocygna autumnalis 11 2. Blue-winged Teal Spatula discors 100 40 6 5 3. Cinnamon Teal Spatula cyanoptera 2 2 23 4. Northern Shoveler Spatula clypeata 100 7 15 4 1 21 5. Gadwall Mareca strepera 6 3 6. American Wigeon Mareca americana 3 7. Northern Pintail Anas acuta 2 8. Green-winged Teal Anas crecca 2 9. Redhead Aythya americana 8 10. Lesser Scaup Aythya affinis 30 11. Ruddy Duck Oxyura jamaicensis 7 7 16 CRACIDAE 12. Rufous-bellied Chachalaca Ortalis wagleri 3 4 2 15 9 4 13. Crested Guan Penelope purpurescens 2 3 2 ODONTOPHORIDAE 14. Elegant Quail Callipepla douglasii 3 1 1 15. Singing Quail Dactylortyx thoracicus H PODICIPEDIDAE 16. Least Grebe Tachybaptus dominicus 3 2 17. Pied-billed Grebe Podilymbus podiceps 2 18. Eared Grebe Podiceps nigricollis 1 4 19. Clark’s Grebe Aechmophorus clarkii 8 COLUMBIDAE 20. Rock Pigeon (I) Columba livia 10 3 5 2 5 10 5 6 1 San Blas & Durango Highway Otus asio Tours 21. Red-billed Pigeon Patagioenis flavirostris 4 12 6 4 8 22. Band-tailed Pigeon Patagioenas fasciata 1 23. Eurasian Collared-Dove Streptopelia decaocto 10 1 2 2 2 2 3 3 2 24. Inca Dove Columbina inca 2 3 2 3 6 1 1 25. Common Ground-Dove Columbina passerina 25 5 26. Ruddy Ground-Dove Columbina talpacoti 20 15 20 1 15 4 20 27. -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

NICK ELLERTON 5 February 1949 – 29 March 2014
NICK ELLERTON 5 February 1949 – 29 March 2014 Obituary Sadly this reports that Nick really knew and cared about cook, a red wine lover and a loyal Ellerton died suddenly in the early animal welfare and was prepared friend. At times he had some morning of Saturday 29 March to be vocal about those issues. He outrageous opinions, strongly 2014, when he suffered a heart always put the needs of the expressed, but was always willing attack, in Sri Lanka. animals first and foremost, even to listen to challenges to those though it made him politically views, even if this did not always Nick worked as Deputy, then unpopular at times. He was a man shift his own. Curator of Mammals at the North ahead of his time in Zoos, and of England Zoological Society particularly drove changes in our Nick was probably the most (Chester Zoo) for 31 years before attitude to elephant welfare and observant person I have ever moving to Knowsley Safari Park for breeding so forcefully back in the known. He noticed everything. 11 years. Together with his 1980s. He was able to get a lot of He felt strongly about looking after longtime partner Penny Boyd progressive ideas around animal his own back yard before (formerly of Burstow Wildlife welfare started and worried little suggesting to other countries how Sanctuary in the UK, and latterly about the fallout for those who to look after theirs. He was also at Knowsley) they moved to were not prepared to change their realistic in the advice he offered to Sri Lanka in the summer of 2010 attitudes. -

Daily Report Monday, 9 November 2020 CONTENTS
Daily Report Monday, 9 November 2020 This report shows written answers and statements provided on 9 November 2020 and the information is correct at the time of publication (07:12 P.M., 09 November 2020). For the latest information on written questions and answers, ministerial corrections, and written statements, please visit: http://www.parliament.uk/writtenanswers/ CONTENTS ANSWERS 8 Licensed Premises: BUSINESS, ENERGY AND Coronavirus 20 INDUSTRIAL STRATEGY 8 Life Sciences 20 Beer: Small Businesses 8 Low Pay: Coronavirus 21 Bounce Back Loan Scheme: Nuclear Power 22 Sussex 8 Nuclear Power Stations: Business: Coronavirus 9 Finance 22 Carbon Emissions 11 Nuclear Reactors 22 Consumer Goods: Safety 11 Overseas Students: EU Coronavirus: Disease Control 12 Nationals 23 Coronavirus: Remote Working 12 Personal Care Services: Coronavirus 23 Coronavirus: Social Distancing 13 Political Parties: Coronavirus 24 Debenhams: Coronavirus 13 Post Office: Legal Costs 24 Economic Situation: Coronavirus 14 Post Offices: ICT 25 Electronic Commerce: Renewable Energy 25 Regulation 14 Research: Public Consultation 27 Energy Supply 15 Research: Publishing 27 Energy: Meters 15 Retail Trade: Coventry 28 Erasmus+ Programme and Shipping: Tees Valley 28 Horizon Europe 16 Solar power: Faversham 29 Fireworks: Safety 16 Unemployment: Coronavirus 29 Green Homes Grant Scheme 17 Weddings: Coronavirus 30 Horizon Europe 18 Wind Power 31 Housing: Energy 19 Hydrogen 20 CABINET OFFICE 31 Musicians: Coronavirus 44 Ballot Papers: Visual Skateboarding: Coronavirus 44 Impairment 31 -

Bird Checklists of the World Country Or Region: Ghana
Avibase Page 1of 24 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Ghana 2 Number of species: 773 3 Number of endemics: 0 4 Number of breeding endemics: 0 5 Number of globally threatened species: 26 6 Number of extinct species: 0 7 Number of introduced species: 1 8 Date last reviewed: 2019-11-10 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Ghana. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=gh [26/09/2021]. Make your observations count! Submit your data to ebird. -

Gear for a Big Year
APPENDIX 1 GEAR FOR A BIG YEAR 40-liter REI Vagabond Tour 40 Two passports Travel Pack Wallet Tumi luggage tag Two notebooks Leica 10x42 Ultravid HD-Plus Two Sharpie pens binoculars Oakley sunglasses Leica 65 mm Televid spotting scope with tripod Fossil watch Leica V-Lux camera Asics GEL-Enduro 7 trail running shoes GoPro Hero3 video camera with selfie stick Four Mountain Hardwear Wicked Lite short-sleeved T-shirts 11” MacBook Air laptop Columbia Sportswear rain shell iPhone 6 (and iPhone 4) with an international phone plan Marmot down jacket iPod nano and headphones Two pairs of ExOfficio field pants SureFire Fury LED flashlight Three pairs of ExOfficio Give- with rechargeable batteries N-Go boxer underwear Green laser pointer Two long-sleeved ExOfficio BugsAway insect-repelling Yalumi LED headlamp shirts with sun protection Sea to Summit silk sleeping bag Two pairs of SmartWool socks liner Two pairs of cotton Balega socks Set of adapter plugs for the world Birding Without Borders_F.indd 264 7/14/17 10:49 AM Gear for a Big Year • 265 Wildy Adventure anti-leech Antimalarial pills socks First-aid kit Two bandanas Assorted toiletries (comb, Plain black baseball cap lip balm, eye drops, toenail clippers, tweezers, toothbrush, REI Campware spoon toothpaste, floss, aspirin, Israeli water-purification tablets Imodium, sunscreen) Birding Without Borders_F.indd 265 7/14/17 10:49 AM APPENDIX 2 BIG YEAR SNAPSHOT New Unique per per % % Country Days Total New Unique Day Day New Unique Antarctica / Falklands 8 54 54 30 7 4 100% 56% Argentina 12 435