Comments to the FDA on Swedish Match
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Talking Tobacco Harm Reduction
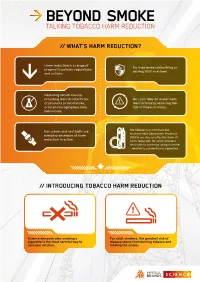
TALKING TOBACCO HARM REDUCTION // WHAT’S HARM REDUCTION? Harm reduction is a range of Do they make sunbathing or pragmatic policies, regulations driving 100% risk-free? and actions. Reducing health risks by providing less harmful forms No – but they do make them of products or substances, less harmful by reducing the or by encouraging less risky risk of illness or injury. behaviours. Sun cream and seat belts are We believe non-combustible nicotine Next Generation Products everyday examples of harm (NGPs) are also an effective form of reduction in action. harm reduction for adult smokers who wish to continue using nicotine – relative to conventional cigarettes. // INTRODUCING TOBACCO HARM REDUCTION Science demonstrates smoking a For adult smokers, the greatest risk of cigarette is the most harmful way to disease stems from burning tobacco and consume nicotine. inhaling the smoke. // WHAT IS THR? Tobacco smoke contains over 7000 The undisputed best action adult chemicals – nicotine is one of them. smokers can take to improve their Around 100 are classified by public health is to stop all tobacco and health experts as causes or potential nicotine use entirely, but many are not causes of smoking-related disease. interested or willing to take this step. Numerous public health bodies1 While the science suggests nicotine is believe transitioning to nicotine addictive and not risk-free, it’s neither products that are substantially less carcinogenic nor the primary cause of harmful than inhaled tobacco smoke smoking-related diseases. is their next best option – we agree. Contains 7000+ Contains chemicals, 100 significantly of them harmful fewer and lower or potentially levels of harmful harmful. -

An Endgame for Tobacco? by the Various Proposals
Editorial Tob Control: first published as 10.1136/tobaccocontrol-2013-050989 on 15 April 2013. Downloaded from then to evaluate what might be achieved An endgame for tobacco? by the various proposals. The latter requires contemplation of each strategy’s Kenneth E Warner potential outcomes, good and undesirable, and the political, legal, ethical, economic, regulatory and social barriers to realising ABSTRACT great successes of many national anti- the strategy’s implementation. Finally, the Since its origins in the 1960s, tobacco control smoking campaigns notwithstanding, the very idea of what constitutes the end of has achieved remarkable success against the pandemic persists. In many developed the endgame needs to be addressed. scourge of tobacco-produced disease and countries with the most robust tobacco Strikingly, to date, there has never been a death. Yet tobacco use, especially cigarette control efforts, the decline in smoking serious discussion within the tobacco smoking, remains the world's leading cause of prevalence has slowed to a trickle. control community about what would preventable premature death and is likely to Evidence-based policies seem incapable of constitute a final victory in tobacco do so for decades to come. Evidence-based substantially hastening the demise of control. Would it be a reduction in 5 policies seem incapable of substantially smoking in these countries. In LMICs, smoking-produced deaths to, say, 10% of hastening the demise of smoking. Slowness in smoking is on the rise. The net effect is current levels? -

A FOCUS on HARM REDUCTION Why It
A FOCUS ON HARM REDUCTION Why it mattersSUSTAINABILITY FOCUS REPORT 2013: How we address the public health impact of our products A nicotine molecule In this report Focusing on the facts 2 At the core of our business strategy 3 Innovative nicotine products 4 ‘Safer’ tobacco products: the research 7 So, what’s next? 9 Our Chief Executive on why it matters b British American Tobacco Harm reduction: sustainability focus report 2013 Because it’s crucial to the future of our business. Surely tobacco harm reduction should just be about What areas are you concentrating on? getting people to quit smoking? Our approach to harm reduction has two distinct areas: The only way to be certain of avoiding the serious health risks nicotine-based alternatives and reduced-risk tobacco products. associated with smoking is not to smoke at all. However, despite In the nicotine category, we have established a stand-alone increasingly strict tobacco control policies, many people continue business solely dedicated to this area. This brings together our to smoke. And the World Health Organisation estimates that existing Nicoventures business with CN Creative, the e-cigarette 1 many more will do so in the future . So realistically the ‘quit or die’ company we acquired at the end of last year, into a single business approach to reducing the public health impact of smoking simply which will continue to operate under the Nicoventures name. isn’t enough. This business has already launched its first e-cigarette in the UK, For adults that choose to continue to smoke, tobacco harm which will be expanded into further markets in the coming year. -

List of References Submitted During the Public Consultation
Bibliography of the Sources Examined for the Opinion on the Addictiveness and Attractiveness of Tobacco Additives ACSCAN (American Cancer Society Cancer Action Network), American Heart Association, American Lung Association, Campaign for Tobacco-Free Kids, American Legacy Foundation. Submission to U.S. Food and Drug Administration re Docket No. FDA- 2010-N-0207, 2010; July 26. Available from: URL: http://www.tobaccofreekids.org/reports/fda/regulatory_docs/Comments%20to%20 FDA%20RFI%20re%20youth%20and%20minority%20marketing%207-26-10.pdf Adam T, McAughey J, McGrath C, Mocker C, Zimmermann R. Simultaneous on-line size and chemical analysis of gas phase and particulate phase of cigarette mainstream smoke. Anal Bioanal Chem 2009; 394:1193-203. Adam T, Mitschke S, Streibel T, Baker RR, Zimmermann R. Puff-by-puff resolved characterisation of cigarette mainstream smoke by single photon ionisation (SPI)- time-of-flight mass spectrometry (TOFMS): comparison of the 2R4F research cigarette and pure Burley, Virginia, Oriental and Maryland tobacco cigarettes. Anal Chim Acta. 2006 Jul 21;572(2):219-29. Epub 2006; May 22. Ahijevych K, Garrett BE. Menthol pharmacology and its potential impact on cigarette smoking behaviour. Nicotine Tob Res 2004; 6 Suppl 1:S17-28. Ahijevych K, Gillespie J, Demirci M, Jagadeesh J. Menthol and nonmenthol cigarettes and smoke exposure in black and white women. Pharmacol Biochem Behav 1996; 53:355-60. Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav 1999; 24:115-20. Akehurst BC Tobacco. Second edition. Tropical Agriculture Series. Longman. London and New York. 1981; Pp. -

Tobacco Control
TOBACCO CONTROL Playbook World Health Organization ABSTRACT Tobacco control is difficult and complex and obstructed by the tactics of the tobacco industry and its allies to oppose effective tobacco control measures. This document was developed by the WHO Regional Office for Europe by collecting numerous evidence-based arguments from different thematic areas, reflecting the challenges that tobacco control leaders have faced while implementing various articles of the WHO FCTC and highlighting arguments they have developed in order to counter and succeed against the tobacco industry. KEY WORDS TOBACCO CONTROL WHO FCTC HEALTH EFFECTS TOBACCO INDUSTRY ARGUMENTS © World Health Organization 2019 All rights reserved. The Regional Office for Europe of the World Health Organization welcomes requests for permission to reproduce or translate its publications, in part or in full. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. -

Tobacco Harm Reduction
Tobacco Harm Reduction Brad Rodu Professor, Department of Medicine James Graham Brown Cancer Center University of Louisville The Smoking Status Quo: Unacceptable • The American Anti-Smoking Campaign is 45 Years Old • According to the CDC: 45 million smokers in the U.S. 443,000 deaths every year in the U.S. 5,800 in Oklahoma Lung Cancer (ICD 161-162) Mortality in Men and Women Age 35+, Oklahoma and the US, 1979-2009 250 OK Men 200 150 US Men OK Women 100 Deaths per 100,000 py 100,000 Deathsper US Women 50 0 Year If the Status Quo Continues In the next 20 years: • 8 million Americans will die from smoking All are adults over 35 years of age None of them are now children The Failed Anti-Smoking Campaign • The Campaign’s Only Message: Quit Nicotine and Tobacco, or Die • The Campaign’s Only Quitting Tactics: Ineffective Behavioral Therapy Ineffective Use of Nicotine Rodu and Cole. Technology 6: 17-21, 1999. Rodu and Cole. International J Cancer 97: 804-806, 2002. The Anti-Smoking Campaign- Behavioral Therapy • NCI Manual for Physicians- Counsel Patients to: – ”Keep your hands busy- doodle, knit, type a letter” – ”Cut a drinking straw into cigarette-sized pieces and inhale air” – ”Keep a daydream ready to go” Source: How to help your patients stop smoking. NIH Pub. No. 93-3064, 1993 The Anti-Smoking Campaign- Faulted Use of Nicotine • Temporary – 6 to 12 weeks • Expensive – per unit and per box • Very Low Dose – unsatisfying for smokers • 7% Success* – ”Efficacious”, ”Modest” *Hughes et al. Meta-analysis in Tobacco Control, 2003. -

Australian Tobacco Harm Reduction Association (ATHRA) Is a Registered Health Promotion Charity Established to Help Reduce the Harm from Tobacco Smoking in Australia
Tobacco Harm Reduction Submission 166 Tobacco Harm Reduction Submission 166 3 November 2020 Select Committee on Tobacco Harm Reduction Dear Senators, Thank you for the opportunity to make a submission to this important Inquiry. Nicotine vaping is the most effective and most popular quitting aid globally. There is overwhelming scientific agreement that vaping nicotine is far safer than smoking and the public health benefits are well recognised. However, Australia remains the only western democracy to ban the sale and use of nicotine for vaping. Countries like the UK and New Zealand treat nicotine liquid as a consumer product and promote vaping as a safer alternative to smoking. No country in the world requires users to have a doctor’s prescription for accessing low concentrations of nicotine. Nicotine vaping is a harm reduction strategy that will complement existing tobacco control and smoking cessation measures and accelerate the decline of smoking in Australia. Australian medical professional associations are gradually acknowledging the benefits of legalised vaping as a public health measure, including the Royal Australian and New Zealand College of Psychiatrists, The Royal Australian College of General Practitioners and the Royal Australasian College of Physicians There is a compelling case to ease restrictions and implement carefully balanced regulations that protect consumers without compromising the considerable potential benefits. Vaping has the potential to improve the lives of hundreds of thousands of Australians, especially disadvantaged and marginalised groups. Board members Dr Joe Kosterich (Chairman) Dr Colin Mendelsohn Dr Alex Wodak AM Mr Ean Alexander What is ATHRA? The Australian Tobacco Harm Reduction Association (ATHRA) is a registered health promotion charity established to help reduce the harm from tobacco smoking in Australia. -

Draft Tobacco Harm Reduction Strategy 2019-23
Havering Health and Wellbeing Board Draft Tobacco Harm Reduction Strategy 2019-23 Introduction Despite rates having reduced over recent years, smoking remains the biggest cause of preventable deaths and the cause of long term health problems for smokers and those exposed to second-hand smoke. Apart from the obvious impact this has on individuals and families, the harms caused by smoking tobacco place extra pressures on health and social care services, damages the environment, negatively impacts productivity in the workplace, and the trade in illegal cigarettes also fuels criminal activity. The Health and Wellbeing Board recognises that tackling the problem of smoking requires system- wide changes and action. By making it a Board priority, this shows that individual members are committed to working together and with partners, in the belief that, through concerted and united efforts, it will be possible to create the right conditions for a smokefree generation of healthier and more prosperous residents. Aim The aim is for the borough to raise a smokefree generation by achieving a smokefree pregnancy for all, recognising that every child deserves the best start in life stopping children from taking up smoking, as it is mostly in childhood that smoking addictions are formed reducing smoking prevalence in adults, paying particular attention to those groups where smoking rates are highest; benefiting individuals that quit and also lowering prevalence and shifting cultural norms in favour of smoke free living protecting the health of non-smokers, by reducing children and adults exposure to second- hand smoke ensuring parity of esteem for those with mental health conditions The approach The following pages set out how tobacco use impacts different groups, and recommends what approaches should be taken. -

Australian Tobacco Harm Reduction Association (ATHRA) Is a Registered Health Promotion Charity Established to Help Reduce the Harm from Tobacco Smoking in Australia
3 November 2020 Select Committee on Tobacco Harm Reduction Dear Senators, Thank you for the opportunity to make a submission to this important Inquiry. Nicotine vaping is the most effective and most popular quitting aid globally. There is overwhelming scientific agreement that vaping nicotine is far safer than smoking and the public health benefits are well recognised. However, Australia remains the only western democracy to ban the sale and use of nicotine for vaping. Countries like the UK and New Zealand treat nicotine liquid as a consumer product and promote vaping as a safer alternative to smoking. No country in the world requires users to have a doctor’s prescription for accessing low concentrations of nicotine. Nicotine vaping is a harm reduction strategy that will complement existing tobacco control and smoking cessation measures and accelerate the decline of smoking in Australia. Australian medical professional associations are gradually acknowledging the benefits of legalised vaping as a public health measure, including the Royal Australian and New Zealand College of Psychiatrists, The Royal Australian College of General Practitioners and the Royal Australasian College of Physicians There is a compelling case to ease restrictions and implement carefully balanced regulations that protect consumers without compromising the considerable potential benefits. Vaping has the potential to improve the lives of hundreds of thousands of Australians, especially disadvantaged and marginalised groups. Board members Dr Joe Kosterich (Chairman) Dr Colin Mendelsohn Dr Alex Wodak AM Mr Ean Alexander What is ATHRA? The Australian Tobacco Harm Reduction Association (ATHRA) is a registered health promotion charity established to help reduce the harm from tobacco smoking in Australia. -

Tobacco Harm Reduction: a Comprehensive Nicotine Policy to Reduce Death and Disease Caused by Smoking (Resolution 403-A-17) (Reference Committee D)
REPORT 5 OF THE COUNCIL ON SCIENCE AND PUBLIC HEALTH (A-18) Tobacco Harm Reduction: A Comprehensive Nicotine Policy to Reduce Death and Disease Caused by Smoking (Resolution 403-A-17) (Reference Committee D) EXECUTIVE SUMMARY Objective: This report examines the available evidence regarding harm reduction approaches to reducing tobacco-related mortality, with a focus on electronic cigarettes. Methods: English language reports were selected from searches of the PubMed, Google Scholar, and Cochrane Library databases from March 2014 to January 2018 using the search terms “tobacco” and “harm reduction,” “nicotine,” “electronic cigarette,” “e-cigarette,” “ENDS,”, “noncombustible tobacco product,” “smokeless tobacco,” and “ tobacco cessation.” Additional articles were identified by manual review of the reference lists of pertinent publications. Recognizing the dynamic nature of the research being published on this topic, the Council deemed it appropriate to summarize the findings and conclusions of the recent National Academies of Sciences, Engineering, and Medicine (National Academies) report on the “Public Health Consequences of E-Cigarettes” related to harm reduction. Articles published subsequent to the National Academies report are cited, as appropriate. Results: Despite reductions in combustible tobacco use, it still represents the leading cause of preventable death in the United States. A growing number of non-combustible tobacco products are thought to be less hazardous than combustibles, but limited evidence is available on their long-term health risks. E-cigarettes are among the most widely used non-combustible tobacco product. Available evidence suggests that those who completely substitute e-cigarettes for combustible tobacco cigarettes have reduced exposure to numerous toxicants and carcinogens present in combustible tobacco cigarettes. -

ÇUKUROVA ÜNİVERSİTESİ SOSYAL BİLİMLER ENSTİTÜSÜ DERGİSİ Journal of Çukurova University Institute of Social Sciences
SSN: 1304 – 8880 Çukurova Üniversitesi Sosyal Bilimler Enstitüsü Çukurova University Institute of Social Sciences ÇUKUROVA ÜNİVERSİTESİ SOSYAL BİLİMLER ENSTİTÜSÜ DERGİSİ Journal of Çukurova University Institute of Social Sciences Cilt/ Vol: 25 Sayı/No: 1 Yıl/Year: 2016 ÇUKUROVA ÜNİVERSİTESİ SOSYAL BİLİMLER ENSTİTÜSÜ DERGİSİ Journal of Çukurova University Institute of Social Sciences Sahibi/Owner Ç.Ü. Sosyal Bilimler Enstitüsü adına Enstitü Müdürü Prof. Dr. Yıldırım B. ÖNAL Derleyen / Managing Editor Prof. Dr. İ. Çetin DERDİYOK Yayın Kurulu / Board of Editors Prof.Dr. İ. Çetin DERDİYOK (Başkan) Prof.Dr. Hasan KAYIKLIK Prof.Dr. Nurçay TÜRKOĞLU Doç.Dr. Meral KILIÇATICI Doç.Dr. Kemal Can KILIÇ Doç.C. Hakan ÇUHADAR Doç.Dr. Ömer KORKUT Yrd.Doç.Dr. Oya AYGÜN Yrd.Doç.Dr. Fatih GÜLŞEN Derleme Sekreteri / Editorial Secretary Doç.Dr. Kemal Can KILIÇ Dizgi-Mizanpaj / Typesetter Seren YOKSULABAKAN Copyright©Nisan 2016 Çukurova Üniversitesi Sosyal Bilimler Enstitüsü Dergisi Tüm hakları mahfuzdur. TÜBİTAK / ULAKBİM Sosyal Bilimler Veri Tabanına (SBVT) dâhildir. Dergimiz EBSCO-CEEAS veri tabanında taranmaktadır. Çukurova Üniversitesi Sosyal Bilimler Enstitüsü Dergisi yılda en az 2 kez yayımlanan hakemli bir dergidir. Dergide yayımlanan makalelerin dil ve bilim sorumluluğu yazara aittir. Makaleler kaynak gösterilmeden kullanılamaz. Elektronik veya mekanik yöntemlerle (fotokopi dahil) herhangi biçimde basılamaz ve / veya çoğaltılamaz. Adres / Address: ÇÜ. Sosyal Bilimler Enstitüsü Müdürlüğü 01330 Balcalı / ADANA Tel: 0 (322) 338 65 74 Faks: 0 (322) 338 69 47 E- Posta: [email protected] İnternet adresi: http://sosyalbilimler.cu.edu.tr Kapak Tasarımı: Metin AYGÜN Baskı: Çukurova Üniversitesi Danışma Kurulu /Advisory Board Prof. Dr. Abdullatif ACARLIOĞLU Anadolu Üniversitesi Prof. Dr. Gül DURMUŞOĞLU KÖSE Anadolu Üniversitesi Prof. -

Harm Reduction Policies for Tobacco
Chapter 9 Harm reduction policies for tobacco Coral Gartner, Wayne Hall and Ann McNeill Abstract Tobacco smoking is the leading cause of preventable premature mortality and disability in European and other developed countries. This chapter first reviews strategies that (1) aim to reduce harm to non-smokers (public smoking bans and reduced ignition propensity cigarettes) and (2) aim to reduce harm to the smoker who is unable or unwilling to quit nicotine use, namely, regulating the harmfulness of cigarettes, and encouraging smokers to switch to less harmful nicotine products. The putative tobacco harm reduction products discussed include: modified tobacco cigarettes and cigarette-like devices, smokeless tobacco products and pharmaceutical nicotine products. The evidence for the harm reduction potential of each of these is discussed, as are adverse public health outcomes that may potentially arise from their promotion. The chapter concludes with a description of the most promising options for promoting tobacco harm reduction. Keywords: smokeless tobacco, snus, reduced ignition propensity cigarettes, smoking bans, potential reduced exposure products, pharmaceutical nicotine. Introduction Tobacco can be smoked as cigarettes, in a pipe, or as cigars or used via non-smoked products such as chewing tobacco or oral and nasal snuff. Nicotine is the primary substance responsible for tobacco dependence but the majority of harm caused by tobacco use is not from nicotine but from the by-products of smoked tobacco (e.g. fine particulates, carcinogens, and noxious gases including carbon monoxide). Cigarettes are the most addictive and hazardous tobacco product, because cigarette smoke is readily drawn deep into the lungs where it is rapidly absorbed into the bloodstream and from which nicotine quickly reaches the brain (Benowitz, 2008).