Pathology of Oesophageal and Gastric Tumours
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endocrine Tumors of the Pancreas
Friday, November 4, 2005 8:30 - 10:30 a. m. Pancreatic Tumors, Session 2 Chairman: R. Jensen, Bethesda, MD, USA 9:00 - 9:30 a. m. Working Group Session Pathology and Genetics Group leaders: J.–Y. Scoazec, Lyon, France Questions to be answered: 12 Medicine and Clinical Pathology Group leader: K. Öberg, Uppsala, Sweden Questions to be answered: 17 Surgery Group leader: B. Niederle, Vienna, Austria Questions to be answered: 11 Imaging Group leaders: S. Pauwels, Brussels, Belgium; D.J. Kwekkeboom, Rotterdam, The Netherlands Questions to be answered: 4 Color Codes Pathology and Genetics Medicine and Clinical Pathology Surgery Imaging ENETS Guidelines Neuroendocrinology 2004;80:394–424 Endocrine Tumors of the Pancreas - gastrinoma Epidemiology The incidence of clinically detected tumours has been reported to be 4-12 per million inhabitants, which is much lower than what is reported from autopsy series (about 1%) (5,13). Clinicopathological staging (12, 14, 15) Well-differentiated tumours are the large majority of which the two largest fractions are insulinomas (about 40% of cases) and non-functioning tumours (30-35%). When confined to the pancreas, non-angioinvasive, <2 cm in size, with <2 mitoses per 10 high power field (HPF) and <2% Ki-67 proliferation index are classified as of benign behaviour (WHO group 1) and, with the notable exception of insulinomas, are non-functioning. Tumours confined to the pancreas but > 2 cm in size, with angioinvasion and /or perineural space invasion, or >2mitoses >2cm in size, >2 mitoses per 20 HPF or >2% Ki-67 proliferation index, either non-functioning or functioning (gastrinoma, insulinoma, glucagonoma, somastatinoma or with ectopic syndromes, such as Cushing’s syndrome (ectopic ACTH syndrome), hypercaliemia (PTHrpoma) or acromegaly (GHRHoma)) still belong to the (WHO group 1) but are classified as tumours with uncertain behaviour. -

Zollinger-Ellison Syndrome. Case Report
https://doi.org/10.15446/cr.v5n1.71686 ZOLLINGER-ELLISON SYNDROME. CASE REPORT Keywords: Gastrinoma; Zollinger-Ellison Sydrome; Multiple Endocrine Neoplasia Type 1. Palabras clave: Gastrinoma; Síndrome de Zollinger-Ellison; Neoplasia endocrina múltiple tipo 1. Juan Felipe Rivillas-Reyes Juan Leonel Castro-Avendaño Universidad Nacional de Colombia - Sede Bogotá - Facultad de Medicina - Programa de Medicina - Bogotá D.C. - Colombia. Héctor Fabián Martínez-Muñoz Universidad Nacional de Colombia - Sede Bogotá - Facultad de Medicina - Departamento de Cirugía - Bogotá D.C. - Colombia. Corresponding author Juan Felipe Rivillas-Reyes. Facultad de Medicina, Universidad Nacional de Colombia. Bogotá D.C. Colombia. Email: [email protected]. Received: 13/04/2018 Accepted: 20/11/2018 zollinger-ellison syndrome ABSTRACT RESUMEN 29 Introduction: The Zollinger-Ellison syndrome Introducción. El síndrome de Zollinger-Elli- (ZES) is a pathology caused by a neuroendo- son (SZE) es una patología producida por un crine tumor, usually located in the pancreas tumor neuroendocrino habitualmente localiza- or the duodenum, which is characterized by do a nivel duodenal o pancreático, el cual pro- elevated levels of gastrin, resulting in an ex- duce niveles elevados de gastrina, derivando cessive production of gastric acid. en hipersecreción de ácido gástrico. Case presentation: A 42-year-old female Presentación del caso. Paciente femenino patient with a history of longstanding peptic de 42 años con antecedente de enfermedad ulcer disease, who consulted due to persistent ulceropéptica de larga data, quién consulta por epigastric pain, melena and signs of peritoneal epigastralgia persistente y deposiciones meléni- irritation. Perforated peptic ulcer was suspect- cas y presenta signos de irritación peritoneal. Se ed, requiring emergency surgical intervention. -

Acute Diarrhea in Adults WENDY BARR, MD, MPH, MSCE, and ANDREW SMITH, MD Lawrence Family Medicine Residency, Lawrence, Massachusetts
Acute Diarrhea in Adults WENDY BARR, MD, MPH, MSCE, and ANDREW SMITH, MD Lawrence Family Medicine Residency, Lawrence, Massachusetts Acute diarrhea in adults is a common problem encountered by family physicians. The most common etiology is viral gastroenteritis, a self-limited disease. Increases in travel, comorbidities, and foodborne illness lead to more bacteria- related cases of acute diarrhea. A history and physical examination evaluating for risk factors and signs of inflammatory diarrhea and/or severe dehydration can direct any needed testing and treatment. Most patients do not require labora- tory workup, and routine stool cultures are not recommended. Treatment focuses on preventing and treating dehydra- tion. Diagnostic investigation should be reserved for patients with severe dehydration or illness, persistent fever, bloody stool, or immunosuppression, and for cases of suspected nosocomial infection or outbreak. Oral rehydration therapy with early refeeding is the preferred treatment for dehydration. Antimotility agents should be avoided in patients with bloody diarrhea, but loperamide/simethicone may improve symptoms in patients with watery diarrhea. Probiotic use may shorten the duration of illness. When used appropriately, antibiotics are effective in the treatment of shigellosis, campylobacteriosis, Clostridium difficile,traveler’s diarrhea, and protozoal infections. Prevention of acute diarrhea is promoted through adequate hand washing, safe food preparation, access to clean water, and vaccinations. (Am Fam Physician. 2014;89(3):180-189. Copyright © 2014 American Academy of Family Physicians.) CME This clinical content cute diarrhea is defined as stool with compares noninflammatory and inflamma- conforms to AAFP criteria increased water content, volume, or tory acute infectious diarrhea.7,8 for continuing medical education (CME). -

Gastrinoma: a Small Tumor with Big Symptoms
Case Report ISSN: 2574 -1241 DOI: 10.26717/BJSTR.2020.31.005159 Gastrinoma: A Small Tumor with Big Symptoms Hira Irfan1, Ahmed Imran Siddiqi1,2*, Waqas Shafiq1 and Umal Azmat1 1Department of Endocrinology, Shaukat Khanum memorial cancer hospital and research center, Lahore, Pakistan 2Department of Medicine Jersey General Hospital, Channel Islands UK *Corresponding author: Ahmed Imran Siddiqi, Department of Endocrinology, Shaukat Khanum memorial cancer hospital and research center, Lahore, Pakistan; Department of Medicine Jersey General Hospital, Channel Islands UK ARTICLE INFO ABSTRACT Received: November 04, 2020 Gastrinoma is rare gastrin secreting tumor and can be challenging to diagnose in the Published: November 11, 2020 absence of typical clinical features. Surgical resection remains the mainstay of treatment and can be curative, but the clinical presentation can compromise complete resection especially if the disease has already metastasized. We present here a 77-year-old lady Citation: Hira Irfan, Ahmed Imran Siddiqi, with abdominal pain and secretory diarrhea. Gastrinoma was diagnosed in a stepwise approach of investigations and then surgically removed. Following surgery, the patient Small Tumor with Big Symptoms. Biomed got prescribed octreotide for complete resolution of symptoms. Conservative treatment JWaqas Sci &Shafiq, Tech Umal Res Azmat.31(5)-2020. Gastrinoma: BJSTR. A is only recommended for patients unsuitable for surgery, with widespread metastasis and MS.ID.005159. on patients’ own choice. Abbreviations: NET: Neuroendocrine Tumors; ZES: Zollinger-Ellison Syndrome; MEN-1: Multiple Endocrine Neoplasia Type-1; SSTRs: Somatostatin Receptors; PTH: Parathyroid Hormone Introduction for a Gastrinoma (5). We report here a case of a 77-year-old lady Gastrinomas are functional neuroendocrine tumors (NET) diagnosed with a Gastrinoma. -
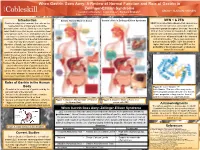
A Review of Normal Function and Role of Gastrin in Zollinger-Ellison
When Gastrin Goes Awry: A Review of Normal Function and Role of Gastrin in Zollinger-Ellison Syndrome Leigha LaTourette1, Janel Cross2, Barbara Brabetz2 Department of Plant and Animal Science1, Department of Natural Sciences and Mathematics2 Introduction Gastrin: Normal Mode of Action Gastrin’s Role in Zollinger-Ellison Syndrome MEN 1 & ZES Gastrin is a digestive hormone that acts on the MEN1 is an inheritable disease that causes over neuroendocrine and parietal cells of the secretion of hormones via tumors on the gastrointestinal tract to ultimately secrete gastric endocrine glands throughout the body.4 Around a acid. Gastrin secretion begins even before food 30% of these tumors are found to be malignant. is consumed, as the mere anticipation of a meal ZES is commonly associated with the MEN1 due can lead to a series of events ending in the to the presence of tumors found in the stomach creation of the gastric acid needed to breakdown and small intestine.4 These gastrointestinal foods. Not only does Gastrin support the tumors caused by MEN 1 give the person a digestion of foods, but it also stimulates growth, higher likelihood of contracting ZES, as the secretion, blood flow, and acts as a defense probability of having pancreatic or duodenal mechanism against bacteria in the tumors is increased.4 gastrointestinal system. When the production of Gastrin becomes overt, major consequences like that of Zollinger Ellison Syndrome (ZES). ZES is an extremely rare disease occurring in people between the ages of 30-60.4 ZES is related to the uncontrolled secretion of gastrin due to the presence of certain pancreatic or duodenal tumors. -

Benign Oesophageal Stricture and Chronic Diarrhoea As Atypical
Open Access Case Report DOI: 10.7759/cureus.16593 Benign Oesophageal Stricture and Chronic Diarrhoea As Atypical Presenting Symptoms of an Advanced Metastatic Pancreatic Gastrinoma: A Case Report and Review of Literature Muhammad Hafiz Kamarul Bahrin 1 , Raoof Hagag 2 , Abuobeida Ali 3 , Seifeldin Yahia 4 , Richard Armstrong 5 1. Gastroenterology, United Lincolnshire Hospitals Trust, Boston, GBR 2. Acute Medicine, United Lincolnshire Hospitals National Health Service (NHS) Trust, Boston, GBR 3. Gastroenterology, Peterborough City Hospital, Peterborough, GBR 4. Diabetes and Endocrinology, United Lincolnshire Hospitals National Health Service (NHS) Trust, Boston, GBR 5. Gastroenterology, United Lincolnshire Hospitals National Health Service (NHS) Trust, Boston, GBR Corresponding author: Muhammad Hafiz Kamarul Bahrin, [email protected] Abstract Gastrinoma or otherwise known as Zollinger-Ellison syndrome is characterised by hypersecretion of gastrin and gastric acid leading to the formation of recurrent atypical ulcers along the upper gastrointestinal tract. It is extremely difficult to diagnose during an acute presentation both due to its rarity and its lack of pathognomonic symptoms. Its symptoms range from mild to severe to life-threatening and often get mistaken for a different condition such as viral gastroenteritis as seen in our case report. The most common symptoms of gastrinoma include abdominal pain, dyspepsia and chronic diarrhoea. It rarely presents as a benign oesophageal stricture with some case series reporting the frequency to be as low as 0.4%. Our literature review of 9 random case reports on gastrinoma/Zollinger-Ellison syndrome selected from Pubmed Central reviewed the frequency of its presenting symptoms and investigation modalities involved throughout its diagnostic process. In summary, it agrees with the findings postulated by Jensen’s series. -

GASTRIC CANCER STRUCTURED REPORTING PROTOCOL (2Nd Edition, 2020)
GASTRIC CANCER STRUCTURED REPORTING PROTOCOL (2nd Edition, 2020) Incorporating the: International Collaboration on Cancer Reporting (ICCR) Carcinoma of the Stomach Dataset www.ICCR-Cancer.org Core Document versions: • ICCR dataset: Carcinoma of the Stomach 1st edition • AJCC Cancer Staging Manual 8th edition • Digestive System Tumours, World Health Organization Classification of Tumours, 5th Edition, Volume 1, 2019 ii Gastric Cancer Structured Reporting Protocol 2nd edition ISBN: 978-1-76081-425-0 Publications number (SHPN): (CI) 200282 Online copyright © RCPA 2020 This work (Protocol) is copyright. You may download, display, print and reproduce the Protocol for your personal, non-commercial use or use within your organisation subject to the following terms and conditions: 1. The Protocol may not be copied, reproduced, communicated or displayed, in whole or in part, for profit or commercial gain. 2. Any copy, reproduction or communication must include this RCPA copyright notice in full. 3. With the exception of Chapter 6 - the checklist, no changes may be made to the wording of the Protocol including any Standards, Guidelines, commentary, tables or diagrams. Excerpts from the Protocol may be used in support of the checklist. References and acknowledgments must be maintained in any reproduction or copy in full or part of the Protocol. 4. In regard to Chapter 6 of the Protocol - the checklist: o The wording of the Standards may not be altered in any way and must be included as part of the checklist. o Guidelines are optional and those which are deemed not applicable may be removed. o Numbering of Standards and Guidelines must be retained in the checklist, but can be reduced in size, moved to the end of the checklist item or greyed out or other means to minimise the visual impact. -

Neuroendocrine TUMORS
NeuroendocrinE This book provides you with five informative chapters These chapters guide the clinician through: • Diagnosing and Treating Gastroenteropancreatic Tumors, Including ICD-9 Codes TumorS • Clinical Presentations and Their Syndromes, Including ICD-9 Codes Diagnosis and Management Diagnosis A Comprehensive Guide to to Guide A Comprehensive NeuroendocrinE The remaining three chapters guide the clinician through the selection of appropriate assays, profiles, and dynamic challenge protocols for diagnosing and monitoring neuroendocrine symptoms. TumorS • Assays, Including CPT Codes A Comprehensive Guide to • Profiles, Including CPT Codes • Dynamic Challenge Protocols, Including CPT Codes Diagnosis and Management Inter Science Institute Inter Science Institute 944 West Hyde Park Boulevard Inglewood, California 90302 (800) 255-2873 (800) 421-7133 (310) 677-3322 Vinik Aaron I. Vinik, MD, PhD Eugene A. Woltering, MD Fax (310) 677-2846 www.interscienceinstitute.com Woltering Thomas M. O’Dorisio, MD Vay Liang W. Go, MD O’Dorisio Go Inter Science Institute GI Council Chairman Eugene A. Woltering, MD, FACS The James D. Rives Professor of Surgery and Neurosciences Chief of the Sections of Surgical Endocrinology and Oncology Director of Surgery Research The Louisiana State University Health Sciences Center New Orleans, Louisiana Executive Members Aaron I. Vinik, MD, PhD, FCP, MACP Professor of Medicine, Pathology and Neurobiology Director of Strelitz Diabetes Research Institute Eastern Virginia Medical School Norfolk, Virginia Vay Liang W. -

Mn 02-104 Deol Q.Qxd
CASE REPORT Solitary Hepatic Gastrinoma Treated With Laparoscopic Radiofrequency Ablation Zöe K. Deol, MD, Eldo Frezza, MD, Steven DeJong, MD, Jack Pickleman, MD ABSTRACT INTRODUCTION Background: This is a case of a solitary hepatic gastri- The annual incidence of the 2 most common neuroen- noma in a 65-year-old male. The patient was diagnosed docrine tumors, insulinoma and gastrinoma, is about 1 with Zollinger-Ellison syndrome in 1991. He had nega- per million.1 Although insulinomas are usually benign, tive radiologic and surgical explorations at that time. He 60% to 80% of gastrinomas are malignant. Hepatic or was maintained on proton-pump inhibitors for the next nodal metastases are found in 60% of patients diagnosed 10 years without symptoms. with Zollinger-Ellison syndrome (ZES).2 Long-term (5- year) survival of patients with resected extrahepatic gas- Methods: A computed tomographic (CT) scan done in trinoma is 95%.3 This number is decreased to 85% in April 2001 demonstrated a 5-cm right hepatic lesion. patients with resected hepatic metastases.4 However, 5- Radionucleotide scanning with octreotide demonstrated year survival in patients with unresected hepatic gastri- intense activity in the same area in the right hepatic lobe. noma is a dismal 30%.2,5,6 Hepatic resection continues to His serum gastrin was 317 pg/mL. He underwent laparo- be an effective treatment for a solitary hepatic gastrino- scopic radiofrequency ablation of the lesion. ma.2 Other methods have been tried in patients with Results: Treatment resulted in a 6-cm ablative area giv- tumor locations or medical problems prohibiting major ing a 1-cm margin on the tumor. -

Specific and Non-Specific Biomarkers in Neuroendocrine
cancers Review Specific and Non-Specific Biomarkers in Neuroendocrine Gastroenteropancreatic Tumors Andrea Sansone 1 , Rosa Lauretta 2, Sebastiano Vottari 3, Alfonsina Chiefari 3, Agnese Barnabei 3 , Francesco Romanelli 1 and Marialuisa Appetecchia 3,* 1 Section of Medical Pathophysiology, Food Science and Endocrinology, Dept. of Experimental Medicine, Sapienza University of Rome, 00165 Rome, Italy 2 Internal Medicine, Angioloni Hospital, San Piero in Bagno, 47026 Forlì-Cesena, Italy 3 Endocrinology Unit, Regina Elena National Cancer Institute IRCCS, Rome 00144, Italy * Correspondence: [email protected] Received: 3 July 2019; Accepted: 2 August 2019; Published: 4 August 2019 Abstract: The diagnosis of neuroendocrine tumors (NETs) is a challenging task: Symptoms are rarely specific, and clinical manifestations are often evident only when metastases are already present. However, several bioactive substances secreted by NETs can be included for diagnostic, prognostic, and predictive purposes. Expression of these substances differs between different NETs according to the tumor hormone production. Gastroenteropancreatic (GEP) NETs originate from the diffuse neuroendocrine system of the gastrointestinal tract and pancreatic islets cells: These tumors may produce many non-specific and specific substances, such as chromogranin A, insulin, gastrin, glucagon, and serotonin, which shape the clinical manifestations of the NETs. To provide an up-to-date reference concerning the different biomarkers, as well as their main limitations, we reviewed and summarized existing literature. Keywords: neuroendocrine tumors; biomarkers; gastroenteropancreatic tumors; prognostic markers; diagnosis 1. Introduction Neuroendocrine tumors (NETs) are a heterogeneous group of rare malignancies which, from a clinical point of view, represent a true challenge for clinicians at all stages of the disease. -

Patterns of GI Tract Injury
Adapted/inspired by: Atlas of Gastrointestinal Pathology. Arnold et al. 2015 Prepared by Kurt Schaberg Last updated: 4/2/2020 Patterns of GI Tract Injury Esophagus Non-keratinizing stratified Normal Esophagus squamous mucosa Benign Incidental Findings: Gastric “Inlet Patch”- Heterotopic Muscularis mucosae gastric mucosa in esophagus Esophageal duct Pancreatic Heterotopia/Metaplasia Glycogenic Acanthosis – Epithelial Submucosal glands hyperplasia with abundant, enlarged superficial glycogenated cells; clinically appears white (Muscularis propria: distal = smooth muscle; proximal = skeletal muscle) Acute Esophagitis Intraepithelial Neutrophils with Erosion/Ulceration GERD Often scattered Eos (usu. < 15/HPF), Intercellular edema, Basal cell hyperplasia, Elongation of vascular papilla. Worse distally (near GEJ). Ulcer! Infections Candida - Look for fungal hyphae, Get PAS-D/GMS HSV - Look for Molding, Multinucleation, Margination in epithelial cells. CMV – Look for inclusions in mesenchymal cells. Medications (“Pill esophagitis”) Look for crystals, resins, and pill fragments; Polarize to help looking for foreign material. Eosinophilic Esophagitis Increased intraepithelial Eosinophils (report per HPF) GERD Eos typically < 15/HPF, Intraepithelial T lymphocytes (“squiggle cells”), Intercellular edema, Basal cell hyperplasia, Elongation of vascular papilla. Worse distally (near GEJ). Eosinophilic Esophagitis Typically >20 Eos/HPF. Often eosinophilic microabscesses with degranulation. Often diffuse or worse proximally. Associated with “Atopic Triad” -

Surgical Management of Zollinger-Ellison Syndrome; State of the Art
Surgical Management of Zollinger-Ellison Syndrome; State of the Art Ellen H. Morrow, MD, JeffreyA. Norton, MD* KEYWORDS Gastrinoma Surgery Diagnosis Localization Outcome MEN-1 Zollinger-Ellison Syndrome (ZES) was originally described in 1955.1 It is a syndrome of acid hypersecretion caused by a gastrin-producing tumor (gastrinoma). Since the recognition of this disease entity, diagnosis and treatment strategies have changed greatly. The purpose of this review is to describe the current standard in diagnosis and surgical management of ZES and to outline some controversies that have arisen recently. EPIDEMIOLOGY The incidence of ZES in the United States is one to three new cases per million per year, making it a rare condition.2 Eighty percent of gastrinomas occur sporadically, while 20% are associated with Multiple Endocrine Neoplasia Type 1 (MEN-1).3 ZES causes 0.1% to 1% of peptic ulcer disease.4 Men are slightly more likely to develop ZES.5 The mean age at which symptoms begin is 41 years.6 Patients with MEN-1– associated ZES are likely to present at a younger age; in the third decade of life.7 ZES occurs in approximately 25% of patients with MEN-1.8 PRESENTATION The presenting signs and symptoms of ZES have also changed with earlier recognition and diagnosis. Initially, it was described as gastric acid hypersecretion, ulcers in unusual locations (jejunum), recurrent ulcers, and nonbeta islet cell tumors of the pancreas.9 Subsequent studies of large numbers of patients with ZES have demonstrated that common presenting symptoms include abdominal pain, diarrhea, heartburn, nausea, and weight loss.6 Diarrhea is a common problem as approximately 80% of patients Division of General Surgery, Department of Surgery, Stanford University School of Medicine, 300 Pasteur Drive, H3591, Stanford, CA 94305-5641, USA * Corresponding author.