GASTRIC CANCER STRUCTURED REPORTING PROTOCOL (2Nd Edition, 2020)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endocrine Tumors of the Pancreas
Friday, November 4, 2005 8:30 - 10:30 a. m. Pancreatic Tumors, Session 2 Chairman: R. Jensen, Bethesda, MD, USA 9:00 - 9:30 a. m. Working Group Session Pathology and Genetics Group leaders: J.–Y. Scoazec, Lyon, France Questions to be answered: 12 Medicine and Clinical Pathology Group leader: K. Öberg, Uppsala, Sweden Questions to be answered: 17 Surgery Group leader: B. Niederle, Vienna, Austria Questions to be answered: 11 Imaging Group leaders: S. Pauwels, Brussels, Belgium; D.J. Kwekkeboom, Rotterdam, The Netherlands Questions to be answered: 4 Color Codes Pathology and Genetics Medicine and Clinical Pathology Surgery Imaging ENETS Guidelines Neuroendocrinology 2004;80:394–424 Endocrine Tumors of the Pancreas - gastrinoma Epidemiology The incidence of clinically detected tumours has been reported to be 4-12 per million inhabitants, which is much lower than what is reported from autopsy series (about 1%) (5,13). Clinicopathological staging (12, 14, 15) Well-differentiated tumours are the large majority of which the two largest fractions are insulinomas (about 40% of cases) and non-functioning tumours (30-35%). When confined to the pancreas, non-angioinvasive, <2 cm in size, with <2 mitoses per 10 high power field (HPF) and <2% Ki-67 proliferation index are classified as of benign behaviour (WHO group 1) and, with the notable exception of insulinomas, are non-functioning. Tumours confined to the pancreas but > 2 cm in size, with angioinvasion and /or perineural space invasion, or >2mitoses >2cm in size, >2 mitoses per 20 HPF or >2% Ki-67 proliferation index, either non-functioning or functioning (gastrinoma, insulinoma, glucagonoma, somastatinoma or with ectopic syndromes, such as Cushing’s syndrome (ectopic ACTH syndrome), hypercaliemia (PTHrpoma) or acromegaly (GHRHoma)) still belong to the (WHO group 1) but are classified as tumours with uncertain behaviour. -

Zollinger-Ellison Syndrome. Case Report
https://doi.org/10.15446/cr.v5n1.71686 ZOLLINGER-ELLISON SYNDROME. CASE REPORT Keywords: Gastrinoma; Zollinger-Ellison Sydrome; Multiple Endocrine Neoplasia Type 1. Palabras clave: Gastrinoma; Síndrome de Zollinger-Ellison; Neoplasia endocrina múltiple tipo 1. Juan Felipe Rivillas-Reyes Juan Leonel Castro-Avendaño Universidad Nacional de Colombia - Sede Bogotá - Facultad de Medicina - Programa de Medicina - Bogotá D.C. - Colombia. Héctor Fabián Martínez-Muñoz Universidad Nacional de Colombia - Sede Bogotá - Facultad de Medicina - Departamento de Cirugía - Bogotá D.C. - Colombia. Corresponding author Juan Felipe Rivillas-Reyes. Facultad de Medicina, Universidad Nacional de Colombia. Bogotá D.C. Colombia. Email: [email protected]. Received: 13/04/2018 Accepted: 20/11/2018 zollinger-ellison syndrome ABSTRACT RESUMEN 29 Introduction: The Zollinger-Ellison syndrome Introducción. El síndrome de Zollinger-Elli- (ZES) is a pathology caused by a neuroendo- son (SZE) es una patología producida por un crine tumor, usually located in the pancreas tumor neuroendocrino habitualmente localiza- or the duodenum, which is characterized by do a nivel duodenal o pancreático, el cual pro- elevated levels of gastrin, resulting in an ex- duce niveles elevados de gastrina, derivando cessive production of gastric acid. en hipersecreción de ácido gástrico. Case presentation: A 42-year-old female Presentación del caso. Paciente femenino patient with a history of longstanding peptic de 42 años con antecedente de enfermedad ulcer disease, who consulted due to persistent ulceropéptica de larga data, quién consulta por epigastric pain, melena and signs of peritoneal epigastralgia persistente y deposiciones meléni- irritation. Perforated peptic ulcer was suspect- cas y presenta signos de irritación peritoneal. Se ed, requiring emergency surgical intervention. -

Gastric Cancer: Surgery and Regional Therapy Epidemiology Risk Factors
Gastric Cancer: Epidemiology Surgery and Regional Therapy Gastric cancer is second leading cause of cancer Timothy J. Kennedy, MD specific mortality world wide (989,600 cases; 738,000 Montefiore Medical Center deaths) accounting for 8% new cancer cases Assistant Professor of Surgery Fourth leading cause of cancer death in the United Upper Gastrointestinal and Pancreas Surgery States (21,320 cases; 10,540 deaths) December 15, 2012 Incidence in Japan 8x higher than US More common in men More common in Asians, blacks, native americans and US hispanics Peak age is 7th decade Incidence of proximal gastric cancer increasing 1 Risk factors Etiology H-pylori infection (90% intestinal type and 30% diffuse type) Exposure to carcinogens (tobacco, salt, nitrites) Pernicious anemia Obesity Adenomatous Polyp Previous gastric surgery Familial history of gastric cancer 4 Molecular Pathogenesis Molecular Pathogenesis Diffuse Type (linitis plastica) Histologic Types (Lauren Classification) Poorly differentiated signet ring cells Intestinal Type (well differentiated) Loss of expression of E-cadherin, a key intercellular Arises from gastric mucosa adhesion molecule which maintains organization of Most common in high risk patient populations epithelial tissue Related to environmental factors Arises within lamina propria of stomach wall and grows in infiltrative/submucosal pattern Associated with older patients and distal tumors Associated with females, younger patients and Incidence is decreasing proximal tumors Better prognosis Associated with early metastases -

Metastasectomy Improves the Survival of Gastric Cancer Patients with Krukenberg Tumors: a Retrospective Analysis of 182 Patients
Cancer Management and Research Dovepress open access to scientific and medical research Open Access Full Text Article ORIGINAL RESEARCH Metastasectomy Improves the Survival of Gastric Cancer Patients with Krukenberg Tumors: A Retrospective Analysis of 182 patients This article was published in the following Dove Press journal: Cancer Management and Research Fuhai Ma 1 Purpose: There is no consensus regarding whether metastasectomy in gastric cancer Yang Li 1 patients with Krukenberg tumors (KTs) is associated with survival benefits. The aim of Weikun Li 1 this study was to evaluate the treatment of KTs of gastric origin in a large series of patients Wenzhe Kang 1 and to identify prognostic factors affecting survival. Hao Liu 1 Patients and Methods: All patients who were diagnosed with gastric cancer and ovarian metastases in a single medical center between January 2006 and December 2016 were Shuai Ma 1 identified and included. The patients were divided into two groups according to treatment Yibin Xie 1 1 modality: a metastasectomy group and a nonmetastasectomy group. Clinicopathological Yuxin Zhong features and overall survival (OS) were compared between the groups. 1 Quan Xu Results: In total, 182 patients were identified; 94 patients presented with synchronous KTs, and 2 Bingzhi Wang 88 developed metachronous KTs during follow-up. OS was significantly longer in the metasta- 2 Liyan Xue sectomy group than in the nonmetastasectomy group among those with synchronous (14.0 1 Yantao Tian months vs 8.0 months; p = 0.001) and metachronous (14 months -

Acute Diarrhea in Adults WENDY BARR, MD, MPH, MSCE, and ANDREW SMITH, MD Lawrence Family Medicine Residency, Lawrence, Massachusetts
Acute Diarrhea in Adults WENDY BARR, MD, MPH, MSCE, and ANDREW SMITH, MD Lawrence Family Medicine Residency, Lawrence, Massachusetts Acute diarrhea in adults is a common problem encountered by family physicians. The most common etiology is viral gastroenteritis, a self-limited disease. Increases in travel, comorbidities, and foodborne illness lead to more bacteria- related cases of acute diarrhea. A history and physical examination evaluating for risk factors and signs of inflammatory diarrhea and/or severe dehydration can direct any needed testing and treatment. Most patients do not require labora- tory workup, and routine stool cultures are not recommended. Treatment focuses on preventing and treating dehydra- tion. Diagnostic investigation should be reserved for patients with severe dehydration or illness, persistent fever, bloody stool, or immunosuppression, and for cases of suspected nosocomial infection or outbreak. Oral rehydration therapy with early refeeding is the preferred treatment for dehydration. Antimotility agents should be avoided in patients with bloody diarrhea, but loperamide/simethicone may improve symptoms in patients with watery diarrhea. Probiotic use may shorten the duration of illness. When used appropriately, antibiotics are effective in the treatment of shigellosis, campylobacteriosis, Clostridium difficile,traveler’s diarrhea, and protozoal infections. Prevention of acute diarrhea is promoted through adequate hand washing, safe food preparation, access to clean water, and vaccinations. (Am Fam Physician. 2014;89(3):180-189. Copyright © 2014 American Academy of Family Physicians.) CME This clinical content cute diarrhea is defined as stool with compares noninflammatory and inflamma- conforms to AAFP criteria increased water content, volume, or tory acute infectious diarrhea.7,8 for continuing medical education (CME). -

Gastrinoma: a Small Tumor with Big Symptoms
Case Report ISSN: 2574 -1241 DOI: 10.26717/BJSTR.2020.31.005159 Gastrinoma: A Small Tumor with Big Symptoms Hira Irfan1, Ahmed Imran Siddiqi1,2*, Waqas Shafiq1 and Umal Azmat1 1Department of Endocrinology, Shaukat Khanum memorial cancer hospital and research center, Lahore, Pakistan 2Department of Medicine Jersey General Hospital, Channel Islands UK *Corresponding author: Ahmed Imran Siddiqi, Department of Endocrinology, Shaukat Khanum memorial cancer hospital and research center, Lahore, Pakistan; Department of Medicine Jersey General Hospital, Channel Islands UK ARTICLE INFO ABSTRACT Received: November 04, 2020 Gastrinoma is rare gastrin secreting tumor and can be challenging to diagnose in the Published: November 11, 2020 absence of typical clinical features. Surgical resection remains the mainstay of treatment and can be curative, but the clinical presentation can compromise complete resection especially if the disease has already metastasized. We present here a 77-year-old lady Citation: Hira Irfan, Ahmed Imran Siddiqi, with abdominal pain and secretory diarrhea. Gastrinoma was diagnosed in a stepwise approach of investigations and then surgically removed. Following surgery, the patient Small Tumor with Big Symptoms. Biomed got prescribed octreotide for complete resolution of symptoms. Conservative treatment JWaqas Sci &Shafiq, Tech Umal Res Azmat.31(5)-2020. Gastrinoma: BJSTR. A is only recommended for patients unsuitable for surgery, with widespread metastasis and MS.ID.005159. on patients’ own choice. Abbreviations: NET: Neuroendocrine Tumors; ZES: Zollinger-Ellison Syndrome; MEN-1: Multiple Endocrine Neoplasia Type-1; SSTRs: Somatostatin Receptors; PTH: Parathyroid Hormone Introduction for a Gastrinoma (5). We report here a case of a 77-year-old lady Gastrinomas are functional neuroendocrine tumors (NET) diagnosed with a Gastrinoma. -

New Jersey State Cancer Registry List of Reportable Diseases and Conditions Effective Date March 10, 2011; Revised March 2019
New Jersey State Cancer Registry List of reportable diseases and conditions Effective date March 10, 2011; Revised March 2019 General Rules for Reportability (a) If a diagnosis includes any of the following words, every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report the case to the Department in accordance with the provisions of N.J.A.C. 8:57A. Cancer; Carcinoma; Adenocarcinoma; Carcinoid tumor; Leukemia; Lymphoma; Malignant; and/or Sarcoma (b) Every New Jersey health care facility, physician, dentist, other health care provider or independent clinical laboratory shall report any case having a diagnosis listed at (g) below and which contains any of the following terms in the final diagnosis to the Department in accordance with the provisions of N.J.A.C. 8:57A. Apparent(ly); Appears; Compatible/Compatible with; Consistent with; Favors; Malignant appearing; Most likely; Presumed; Probable; Suspect(ed); Suspicious (for); and/or Typical (of) (c) Basal cell carcinomas and squamous cell carcinomas of the skin are NOT reportable, except when they are diagnosed in the labia, clitoris, vulva, prepuce, penis or scrotum. (d) Carcinoma in situ of the cervix and/or cervical squamous intraepithelial neoplasia III (CIN III) are NOT reportable. (e) Insofar as soft tissue tumors can arise in almost any body site, the primary site of the soft tissue tumor shall also be examined for any questionable neoplasm. NJSCR REPORTABILITY LIST – 2019 1 (f) If any uncertainty regarding the reporting of a particular case exists, the health care facility, physician, dentist, other health care provider or independent clinical laboratory shall contact the Department for guidance at (609) 633‐0500 or view information on the following website http://www.nj.gov/health/ces/njscr.shtml. -

Primary Signet-Ring Cell Linitis Plastica Type Adenocarcinoma of the Urinary Bladder
CaSe report primary signet-ring cell linitis plastica type adenocarcinoma of the urinary bladder yajvender pratap Singh rana1, dharamveer Singh1, Sanjay kumar gupta1, aditya pradhan1, raghav Talwar1, reena bhardwaj2, uday Miglani1, Shalabh agarwal1, Mahesh Chandra1, Sandeep Harkar1, yogesh kumar Swami1, Shafi wani1 1Department of Urology, Army Hospital, Research & Referral, Delhi, India 2Department of Pathology, Army Hospital, Research & Referral, Delhi, India enhanced computer tomography (CECT) of the abdomen showed key wordS diffuse thickening of bladder wall with perivesical fat stranding, linitis plastica » adenocarcinoma » urinary bilateral hydroureteronephrosis (left >right), multiple enlarged bladder » lower urinary tract symptoms pelvic lymph nodes (Fig. 1). She was further evaluated with cys- topanendoscopy (CPE); findings were: small contracted bladder, bilateral ureteric orifices were not seen, and bladder mucosa abStraCt was thickened. Bladder biopsy was taken from multiple sites. Adenocarcinoma of the urinary bladder is unusual, The histopathology (HPE) report showed poorly differentiated constituting about 2% of all primary carcinomas of the adenocarcinoma of the urinary bladder with signet-ring cell bladder. Bladder adenocarcinoma consisting chiefly of features. signet ring cells and those that have a linitis plastica Her course of illness was complicated by issues that she pattern of infiltration is extremely rare. Not many cases developed contrast induced nephropathy (s-creatinine – 4.5 have been reported in literature. To the best of our mg%), right hemi-paresis, and aphasia. Neurological involve- knowledge less than 100 cases of signet-ring cell adeno- ment created a suspicion that she had brain metastasis. Whole carcinoma of the urinary bladder have been reported body PET scan showed an FDG (fluorodeoxyglucose)-avid and that of linitis plastica are even less. -

Gastric Linitis Plastica
Gastric linitis plastica Author: Doctor Marc Pocard1 Creation Date: September 2002 Scientific Editor: Professor. Jean-Alain Chayvialle 1Service de chirurgie digestive, Institut Gustave Roussy, 39 Rue Camille Desmoulins, 94805 Villejuif Cedex, France. [email protected] Abstract Keywords Definition Diagnosis Etiology Epidemiology Particularity of evolution Treatment References Abstract Gastric linitis plastica is a very particular malignant gastric tumor different from the usual gastric adenocarcinoma. Linitis plastica refers to the diffuse proliferation of the connective tissue, resulting in tissue thickening so that the stomach is constricted and rigid. Pathological exams reported a strong connective stroma-reaction associated with a malignant glandular proliferation of independent cells (signet-ring cells), invading all the layers of the digestive tract, the mucosa being usually save not affected.Diagnosis is based on the association of pathological results findings revealed by endoscopic, endoscopic ultrasonography, radiological and surgical examinations. Opposed to the adenocarcinoma, Helicobacter Pylori seems not to be associated with the occurrence of gastric linitis. Familial forms of gastric linitis and breast cancer-associated forms have been reported. Treatment of gastric linitis without carcinomatosis is based on surgical resection, mainly a total gastrectomy. However, prognosis is poor, leading some surgeons to question the interest of such resection. A chemotherapy is usually offered to the patient, but no guideline has been really established, and results are also variable. Keywords Malignant gastric tumor, connective stroma-reaction, malignant glandular proliferating cells. Definition reference examination enabling the detection of Linitis plastica refers to the diffuse proliferation of localized lesion, thus changing the classical the connective tissue, resulting in tissue aspect of complete gastric involvement of gastric thickening so that the stomach is constricted and linitis. -
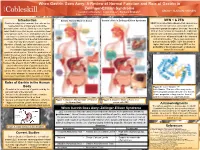
A Review of Normal Function and Role of Gastrin in Zollinger-Ellison
When Gastrin Goes Awry: A Review of Normal Function and Role of Gastrin in Zollinger-Ellison Syndrome Leigha LaTourette1, Janel Cross2, Barbara Brabetz2 Department of Plant and Animal Science1, Department of Natural Sciences and Mathematics2 Introduction Gastrin: Normal Mode of Action Gastrin’s Role in Zollinger-Ellison Syndrome MEN 1 & ZES Gastrin is a digestive hormone that acts on the MEN1 is an inheritable disease that causes over neuroendocrine and parietal cells of the secretion of hormones via tumors on the gastrointestinal tract to ultimately secrete gastric endocrine glands throughout the body.4 Around a acid. Gastrin secretion begins even before food 30% of these tumors are found to be malignant. is consumed, as the mere anticipation of a meal ZES is commonly associated with the MEN1 due can lead to a series of events ending in the to the presence of tumors found in the stomach creation of the gastric acid needed to breakdown and small intestine.4 These gastrointestinal foods. Not only does Gastrin support the tumors caused by MEN 1 give the person a digestion of foods, but it also stimulates growth, higher likelihood of contracting ZES, as the secretion, blood flow, and acts as a defense probability of having pancreatic or duodenal mechanism against bacteria in the tumors is increased.4 gastrointestinal system. When the production of Gastrin becomes overt, major consequences like that of Zollinger Ellison Syndrome (ZES). ZES is an extremely rare disease occurring in people between the ages of 30-60.4 ZES is related to the uncontrolled secretion of gastrin due to the presence of certain pancreatic or duodenal tumors. -

Appearance of Krukenberg Tumor from Gastric Carcinoma, US and CT Evaluation
Short Communication Clinics in Oncology Published: 08 Jul, 2018 Appearance of Krukenberg Tumor from Gastric Carcinoma, US and CT Evaluation Antonio Gligorievski* University Clinic of Surgery "St. Naum Ohridski" Skopje, Macedonia Abstract Introduction: The Krukenberg tumor is a rare malignant tumor of the ovary, accounting from 1% to 2% of all ovarian tumors. It is usually a bilateral involvement of ovaries from the metastatic deposit from adenocarcinoma of the stomach. Patients and Methods: We present a patient at the age of 45, who visited a doctor because of pain in the stomach, nausea, and vomiting and weight loss of more than 20 kg. Clinical, biochemical, endoscopic, pathohistological, radiological and imaging studies (US and CT) have been performed. The radiological examination was performed using a monocontrast and double-contrast technique. The US examination was performed with a 3.75 MHz convex probe, using a standard overview technique. CT was made after oral administration of 700 ml of water, i.v. application of a non-ionic contrast agent of 90 ml in bolus, in hypotonia achieved by i.v. glucagon application, with a scanning width of 10 mm. Presentation of the Case: The radiological finding is in favor of diffuse neoplastic submucosal infiltration of the stomach wall in the region of the corpus and antrum with expressed desmoplastic reaction (linitis plastica). CT findings confirm the radiological findings of the stomach; clearly visualize metastases in the ovaries and peritoneum-ascites, thus diagnosing the primary inoperable neoplastic stomach process - linitis plastica, with metastases in the ovaries - Krukenberg tumor. A comparison of the obtained radiological and imaging results with the endoscopic and pathohistological findings has been made. -

Benign Oesophageal Stricture and Chronic Diarrhoea As Atypical
Open Access Case Report DOI: 10.7759/cureus.16593 Benign Oesophageal Stricture and Chronic Diarrhoea As Atypical Presenting Symptoms of an Advanced Metastatic Pancreatic Gastrinoma: A Case Report and Review of Literature Muhammad Hafiz Kamarul Bahrin 1 , Raoof Hagag 2 , Abuobeida Ali 3 , Seifeldin Yahia 4 , Richard Armstrong 5 1. Gastroenterology, United Lincolnshire Hospitals Trust, Boston, GBR 2. Acute Medicine, United Lincolnshire Hospitals National Health Service (NHS) Trust, Boston, GBR 3. Gastroenterology, Peterborough City Hospital, Peterborough, GBR 4. Diabetes and Endocrinology, United Lincolnshire Hospitals National Health Service (NHS) Trust, Boston, GBR 5. Gastroenterology, United Lincolnshire Hospitals National Health Service (NHS) Trust, Boston, GBR Corresponding author: Muhammad Hafiz Kamarul Bahrin, [email protected] Abstract Gastrinoma or otherwise known as Zollinger-Ellison syndrome is characterised by hypersecretion of gastrin and gastric acid leading to the formation of recurrent atypical ulcers along the upper gastrointestinal tract. It is extremely difficult to diagnose during an acute presentation both due to its rarity and its lack of pathognomonic symptoms. Its symptoms range from mild to severe to life-threatening and often get mistaken for a different condition such as viral gastroenteritis as seen in our case report. The most common symptoms of gastrinoma include abdominal pain, dyspepsia and chronic diarrhoea. It rarely presents as a benign oesophageal stricture with some case series reporting the frequency to be as low as 0.4%. Our literature review of 9 random case reports on gastrinoma/Zollinger-Ellison syndrome selected from Pubmed Central reviewed the frequency of its presenting symptoms and investigation modalities involved throughout its diagnostic process. In summary, it agrees with the findings postulated by Jensen’s series.