The Helix Model of Innovation in Israel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 | Page Danae Christodoulou, US Food and Drug Administration
Bios Danae Christodoulou, US Food and Drug Administration [email protected] Danae Christodoulou is an Acting Branch Chief in the Office of New Drug Products/OPQ/CDER. Danae joined FDA in 1998 and served as primary reviewer in the Office of New Drugs, as a Chemistry, Manufacturing and Controls Lead and Acting Branch Chief since 2013. Danae has a background in Inorganic Chemistry and received her Ph.D. from the University of Michigan, Ann Arbor, MI. Prior to FDA, Danae worked at Johnson Matthey Inc. R&D Drug Discovery as a Senior Research Chemist and at the National Cancer Institute, in Frederick, MD. Danae served as the Chair for the EI Implementation Working Group at FDA. Marjorie Coppinger, Teva [email protected] Marjorie Coppinger is Head of Global Generic Research Quality (SOD/SSL) for Teva Pharmaceuticals. She received B.A in Chemistry from the University of Notre Dame, Maryland and has over 21 years of experience at Teva Pharmaceuticals in analytical research and development, technical services, manufacturing, quality control, compendial compliance and quality assurance. Currently Ms. Coppinger is Co- Chair of the NJPQCA Compendial Discussion Group and has represented both NJPQCA and GPhA on various USP planning meetings, stakeholder initiatives and project groups for the USP. Elisabeth Corbett, Bristol-Myers Squibb. [email protected] Elisabeth Corbett is an Associate Director, CMC, in Global Regulatory Sciences at Bristol-Myers Squib in Pennington, NJ. She joined Bristol-Myers Squibb as a process engineer in Process Chemistry R&D in 2001. In 2008, she relocated to San Antonio, Texas and served in Quality roles before returning to CMC Regulatory at BMS in 2012. -

Celebrating 20 Years of SPECT/CT the Introduction of Hawkeye, the World’S First Hybrid Imaging System
CLINICAL VALUE HAWKEYE SPECT/CT Celebrating 20 Years of SPECT/CT The introduction of Hawkeye, the world’s first hybrid imaging system Nearly 20 years ago, a pivotal event shaped the future of The unclear medicine nuclear medicine. Once called the “unclear medicine,” nuclear Invented by Hal O. Anger in the mid-1950s, nuclear medicine medicine was forever changed with the introduction of cameras detect gamma radiation to depict metabolic Hawkeye, the world’s first clinical hybrid imaging system. processes within a living body. While gamma cameras helped GE Healthcare (at that time known as GE Medical Systems) revolutionize the field of functional medical imaging, there was introduced Hawkeye, a dual-modality SPECT and CT system, at one key limitation: the lack of anatomical markers in the image. the 1999 Society of Nuclear Medicine (SNM) Annual Meeting, heralding in a new era of “more clear medicine.” “We always understood that we needed anatomical and structural data to provide the best details and a precise answer to the clinical question,” says Ora Israel, MD, Director Emeritus of Nuclear Medicine/PET (retired) at Rambam Healthcare Campus, Haifa, Israel. She recalls using fiducial markers placed on the patient’s body to identify the location of key anatomical areas, such as the chest or abdomen. In some cases, Professor Israel and her colleagues would try to repeat the nuclear medicine study on a CT with the patient in the same position. It was a difficult and time-consuming process that she says never really worked well. “There was a clear purpose to improve the specificity of nuclear medicine by defining the anatomical relationship between isotope imaging and anatomy,” says Martin Sandler, MD, Professor of Radiology and Radiological Sciences and formerly Chairman of the Department of Radiology and Radiological Sciences at Vanderbilt University School of Medicine. -
Teva to Acquire Cogenesys
TEVA PHARMACEUTICALS AND PERRIGO COMPANY ANNOUNCE THE U.S. LAUNCH OF GENERIC TEMOZOLOMIDE Jerusalem and Allegan, Mich. – August 12, 2013 – Teva Pharmaceutical Industries Ltd. (NYSE: TEVA) and Perrigo Company (NYSE: PRGO;TASE) today announced the launch of the generic equivalent to Temodar® (temozolomide). Teva will manufacture, market and distribute the product in the U.S. and both companies will equally share in the cost and profitability of the product in the U.S. Teva was first to file, making the product eligible for 180 days of marketing exclusivity. This product is the generic equivalent to Temodar® (temozolomide), indicated for the treatment of adult patients with newly diagnosed glioblastoma multiforme concomitantly with radiotherapy and then as maintenance treatment and refractory anaplastic astrocytoma patients who have experienced disease progression on a drug regimen containing nitrosourea and procarbazine. Temodar® had annual sales of approximately $423 million in the United States, according to IMS data as of December 31, 2012. The launch of this product provides a quality alternative, making cancer therapy more cost effective for patients who suffer from this devastating cancer. Perrigo’s Chairman, President and CEO Joseph C. Papa stated, “This first-to-file launch with our partner Teva is another example of our focus to manufacture complex API’s. We are pleased to offer this important product to patients in the United States.” “We are pleased to partner with Perrigo to offer patients a high-quality, less expensive alternative of this important medicine. This launch demonstrates Teva’s commitment to continue to pursue first-to-market opportunities and enhance the value of our portfolio by concentrating on high-margin, low competition markets,” stated Allan Oberman, President and CEO of Teva Americas Generics. -

Teva Respiratory LLC V. Perrigo Pharmaceuticals
Case 1:20-cv-00207 ECF No. 1 filed 03/09/20 PageID.1 Page 1 of 21 IN THE UNITED STATES DISTRICT COURT FOR THE WESTERN DISTRICT OF MICHIGAN ) ) TEVA RESPIRATORY, LLC, and TEVA ) PHARMACEUTICALS USA, INC., ) ) Civil Action No. 20-00207 ) Plaintiffs, ) ) v. ) ) PERRIGO PHARMACEUTICALS CO., and ) PERRIGO COMPANY PLC, ) ) Defendants. ) ) ) COMPLAINT Plaintiffs Teva Respiratory, LLC (“Teva Respiratory”) and Teva Pharmaceuticals USA, Inc. (“Teva USA”) (collectively, “Plaintiffs” or “Teva”), allege against defendants Perrigo Pharmaceuticals Co., and Perrigo Company plc (collectively, “Defendants” or “Perrigo”) as follows: INTRODUCTION 1. This case arises from Perrigo’s deliberate and unlawful attempt to misappropriate Teva’s federally registered trade dress for Teva’s revolutionary ProAir RespiClick® rescue respiratory inhaler and usurp and tarnish Teva’s goodwill. 2. Teva’s ProAir RespiClick® is the only albuterol rescue inhaler on the market that is “breath-activated”; it does not require any hand-breath coordination during inhalation. In other words, Teva’s ProAir RespiClick® enables users to get a rescue dose of albuterol during an asthma or COPD attack simply by breathing in. ProAir RespiClick® is a leading drug for the treatment of bronchospasm. -1- ACTIVE/102581507.4 Case 1:20-cv-00207 ECF No. 1 filed 03/09/20 PageID.2 Page 2 of 21 3. Teva’s ProAir RespiClick® design includes a white body and a distinctive red cap. Teva has a federally registered trademark on the ProAir RespiClick® design. 4. Teva also sells an earlier-generation albuterol rescue inhaler named ProAir® HFA, both as a branded product and as an authorized generic product. -

4.Employment Education Hebrew Arnona Culture and Leisure
Did you know? Jerusalem has... STARTUPS OVER OPERATING IN THE CITY OVER SITES AND 500 SYNAGOGUES 1200 39 MUSEUMS ALTITUDE OF 630M CULTURAL INSTITUTIONS COMMUNITY 51 AND ARTS CENTERS 27 MANAGERS ( ) Aliyah2Jerusalem ( ) Aliyah2Jerusalem JERUSALEM IS ISRAEL’S STUDENTS LARGEST CITY 126,000 DUNAM Graphic design by OVER 40,000 STUDYING IN THE CITY 50,000 VOLUNTEERS Illustration by www.rinatgilboa.com • Learning centers are available throughout the city at the local Provide assistance for olim to help facilitate a smooth absorption facilities. The centers offer enrichment and study and successful integration into Jerusalem. programs for school age children. • Jerusalem offers a large selection of public and private schools Pre - Aliyah Services 2 within a broad religious spectrum. Also available are a broad range of learning methods offered by specialized schools. Assistance in registration for municipal educational frameworks. Special in Jerusalem! Assistance in finding residence, and organizing community needs. • Tuition subsidies for Olim who come to study in higher education and 16 Community Absorption Coordinators fit certain criteria. Work as a part of the community administrations throughout the • Jerusalem is home to more than 30 institutions of higher education city; these coordinators offer services in educational, cultural, sports, that are recognized by the Student Authority of the Ministry of administrative and social needs for Olim at the various community Immigration & Absorption. Among these schools is Hebrew University – centers. -

Wellbutrin Xl : Civil Action Antitrust Litigation : : : No
Case 2:08-cv-02433-GAM Document 419 Filed 05/11/12 Page 1 of 95 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA IN RE: WELLBUTRIN XL : CIVIL ACTION ANTITRUST LITIGATION : : : NO. 08-2431 (direct) : NO. 08-2433 (indirect) MEMORANDUM McLaughlin, J. May 11, 2012 TABLE OF CONTENTS Page I. Legal and Factual Background.. 2 A. The Drug Approval Process and Regulatory Framework. 2 B. The Citizen Petition Process. 4 C. Wellbutrin IR, Wellbutrin SR, and Wellbutrin XL.. 5 D. Noerr-Pennington Immunity and the Sham Exception. 7 E. Standard of Proof as to Objective Baselessness. 11 II. Overview.. 13 III. Biovail’s Conduct. 15 A. The Anchen Lawsuit. 15 B. The Watson Lawsuit. 26 C. The Abrika Lawsuit. 28 D. The Impax Lawsuit.. 43 E. The Citizen Petition. 50 IV. GSK’s Conduct. 82 A. The Impax and Watson Lawsuits.. 84 B. Biovail’s Citizen Petition. 86 Case 2:08-cv-02433-GAM Document 419 Filed 05/11/12 Page 2 of 95 IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA IN RE: WELLBUTRIN XL : CIVIL ACTION ANTITRUST LITIGATION : : : NO. 08-2431 (direct) : NO. 08-2433 (indirect) MEMORANDUM McLaughlin, J. May 11, 2012 Wellbutrin XL is a once-a-day antidepressant containing the active pharmaceutical ingredient bupropion hydrochloride. It is produced by Biovail Corporation, Biovail Laboratories, Inc., and Biovail Laboratories International SRL1 (together, “Biovail”), and distributed by SmithKline Beecham Corporation and GlaxoSmithKline, PLC (together, “GSK”). The plaintiffs, direct and indirect purchasers of Wellbutrin XL, have sued Biovail and GSK for illegally conspiring to prevent generic versions of Wellbutrin XL from entering the American market by filing sham patent infringement lawsuits and a citizen petition with the Food and Drug Administration (“FDA”), and entering into agreements with generic companies to settle the lawsuits. -

2020 Annual Report Products
Products 2020 Annual Report From the CEO Dear Fellow Shareholders, Perrigo’s transformation to a pure-play Consumer Self-Care Company has come a long way LQMXVWWZRVKRUW\HDUV:HKDYHUHVWRUHGVXVWDLQDEOHWRSOLQHJURZWKGHOLYHUHGRQRXU¿QDQFLDO SURPLVHVUHFRQ¿JXUHGRXUSRUWIROLRRIEXVLQHVVHVXSGDWHGWKH,7LQIUDVWUXFWXUHDQGSURFHVVHV of the Company, expanded capacity, upgraded leadership talent, installed business intelligence capabilities, built a new product pipeline of over $500 million and re-instilled a sense of pride and energy among our 11,000 team members. Making this even more remarkable, is that we kept the WUDQVIRUPDWLRQRQWUDFNLQWKHIDFHRIWKHJOREDO&29,'SDQGHPLF,KRSH\RXDUHDVSURXGRI3HUULJR¶VJOREDOWHDP DV,DPIRUKRZWKH\ZRUNHGWRNHHSHDFKRWKHUVDIHNHSWRXUHVVHQWLDOSURGXFWVÀRZLQJDQGNHSWRXUWUDQVIRUPDWLRQ to a consumer self-care company on track through all of the personal and professional uncertainty that came their way LQ7KH\DUHKHURHV As a result of their efforts, Perrigo delivered strong net sales growth for the second year in a row in 2020 and World-wide Consumer sales reached a new record high. Equally important, the team stabilized adjusted operating income after a few years of decline even as we invested over $50 million in our business and overcame $35 million RIXQSODQQHGKHDGZLQGVGXHSULPDULO\WR&29,'UHODWHGVDIHW\FRVWVDQGEXVLQHVVLPSDFWIURPWKHZHDNFROG FRXJKDQGÀXVHDVRQUHODWHGWR&29,'¶VLPSDFWRQSXEOLFOLIH$OOLQDOOZHKDGDYHU\VWURQJ\HDU Our transformation efforts reached an essential milestone after the year closed when we announced the sale of RXU3UHVFULSWLRQ3KDUPDFHXWLFDOVEXVLQHVVWR$OWDULV&DSLWDO3DUWQHUV//&7KHWUDQVDFWLRQUHLQIRUFHVRXUDELOLW\ -

Foreign Private Issuers Lists, 1996
u.s. Securities and Exchange Commission FOREIGN COMPANIES REGISTERED AND REPORTING WITH THE u.S. SECURITIES AND EXCHANGE COMMISSION DECEMBER 31 , 1996 Office of International Corporate Finance Division of Corporation Finance REPORTING FOREIGN ISSUERS AS OF DECEMBER 31, 1996 SUMMARY"INFORMATION REPORTING COUNTRY COMPANIES CANADA 374 UNITED KINGDOM 81 ISRAEL 71 MEXICO 30 NETHERLANDS 29 AUSTRALIA 26 BERMUDA 22 CHILE 22 JAPAN 21 FRANCE 18 ITALY 13 ARGENTINA 12 BRITISH VIRGIN ISLANDS 11 IRELAND 11 SWEDEN 10 INDONESIA 9 GERMANY 8 LUXEMBOURG 8 SPAIN 8 NETHERLANDS ANTILLES 7 NORWAY 7 BAHAMAS 6 SOUTH AFRICA 6 CHINA 5 FINLAND 5 HONG KONG 5 LIBERIA 5 BRAZIL 4 CAYMAN ISLANDS 4 COLOMBIA 4 DENMARK 4 KOREA 4 NEW ZEALAND 4 VENEZUELA 4 PERU 3 PORTUGAL 3 SINGAPORE 3 BELGIUM 2 PANAMA 2 PHILIPPINES 2 BELIZE 1 BOTSWANA 1 GHANA 1 PAPUA NEW Gl.!lNEA 1 RUSSIA 1 SWITZERLAND 1 TAIWAN 1 ZAMBIA 1 TOTAL 881 p MARKET SUMMARY BY COUNTRY NASDAQ NASDAQ COUNTRY NYSE AMEX NMS Small Cap OTC COMBINED ARGENTINA 10 0 1 0 1 12 26 AUSTRALIA 9 0 7 3 7 BAHAMAS 1 0 2 0 3 6 BELGIUM 0 0 2 0 0 2 BELIZE 0 0 1 0 0 1 BERMUDA 9 3 7 1 2 22 BOTSWANA 0 0 0 0 1 1 4 BRAZIL 2 0 1 0 1 BRIT. V.1. 0 0 8 1 2 11 CANADA 61 39 94 57 123 374 CAYMAN ISLANDS 0 2 2 0 0 4 CHILE 19 0 1 0 2 22 CHINA 5 0 0 0 0 5 COLOMBIA 2 0 0 0 2 4 DENMARK 3 0 1 0 0 4 FINLAND 3 '0 0 1 1 5 FRANCE 9 0 6 0 3 18 GERMANY 5 0 1 0 2 8 GHANA 1 0 0 0 0 1 HONG KONG 1 0 0 0 4 5 0 4 9 I INDONESIA 4 0 1 IRELAND 5 0 3 1 2 11 ISRAEL 4 5 45 11 6 71 ITALY 11 0 2 0 0 13 JAPAN 11 0 7 1 2 21 KOREA 3 0 0 0 1 4 LIBERIA 2 2 0 0 1 5 LUXEMBOURG 3 0 5 0 0 8 MEXICO 25 2 0 0 3 30 NETH. -

Ground to a Halt, Denial of Palestinians' Freedom Of
Since the beginning of the second intifada, in September 2000, Israel has imposed restrictions on the movement of Palestinians in the West Bank that are unprecedented in scope and duration. As a result, Palestinian freedom of movement, which was limited in any event, has turned from a fundamental human right to a privilege that Israel grants or withholds as it deems fit. The restrictions have made traveling from one section to another an exceptional occurrence, subject to various conditions and a showing of justification for the journey. Almost every trip in the West Bank entails a great loss of time, much uncertainty, friction with soldiers, and often substantial additional expense. The restrictions on movement that Israel has imposed on Palestinians in the West Bank have split the West Bank into six major geographical units: North, Central, South, the Jordan Valley and northern Dead Sea, the enclaves resulting from the Separation Barrier, and East Jerusalem. In addition to the restrictions on movement from area to area, Israel also severely restricts movement within each area by splitting them up into subsections, and by controlling and limiting movement between them. This geographic division of the West Bank greatly affects every aspect of Palestinian life. B’TSELEM - The Israeli Information Center for Human Rights in the Occupied Territories Ground to a Halt 8 Hata’asiya St., Talpiot P.O. Box 53132 Jerusalem 91531 Denial of Palestinians’ Freedom Tel. (972) 2-6735599 Fax. (972) 2-6749111 of Movement in the West Bank www.btselem.org • [email protected] August 2007 Ground to a Halt Denial of Palestinians’ Freedom of Movement in the West Bank August 2007 Stolen land is concrete, so here and there calls are heard to stop the building in settlements and not to expropriate land. -
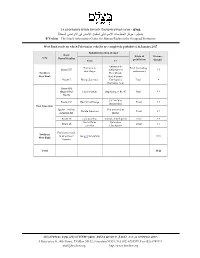
Forbidden Roads Table
בצלם - מרכז המידע הישראלי לזכויות האדם בשטחים (ע.ר.) بتسيلم - مركز المعلومات اﻹسرائيلي لحقوق اﻹنسان في اﻷراضي المحتلة B’Tselem – The Israeli Information Center for Human Rights in the Occupied Territories West Bank roads on which Palestinian vehicles are completely prohibited, 31 January 2017 Prohibited section of road Road Scope of Distance Area Name/Number prohibition From To (in km) Entrance to Entrance to Total (including Route 557 settlement of 3.9 Beit Dajan pedestrians) Northern Elon Moreh West Bank Kafr Kassem Route 5 Bruqin Junction Checkpoint, Total 4 the Green Line Route 404 (Begin Blvd. Har Hotzvim Beginning of Rt. 45 Total 5.8 North) Giv’at Ze’ev Route 443 Beit ‘Ur al-Fauqa Total 7.8 intersection East Jerusalem Qedar – Ma’ale Old entrance to Ma’ale Adumim Total 4.2 Adumim Rd. Qedar Route 60 Gilo Junction Tunnels Checkpoint Total 4.6 Giv’at Ze’ev Qalandiya Route 45 Total 3.2 Junction Checkpoint Prohibited roads Southern in downtown See p. 3 for details 6.72 West Bank Hebron Total 40.22 רחוב התעשייה 8, ת.ד. 53132, ירושלים 91531, טלפון 6735599 (02), פקס 6749111 (02) 8 Hata’asiya St. (4th Floor), P.O.Box 53132, Jerusalem 91531, Tel. (02) 6735599, Fax (02) 6749111 [email protected] http://www.btselem.org West Bank roads on which Palestinian vehicles are restricted, 31 January 2017 Prohibited section of road Road Scope of Distance Area Name/Number prohibition (in km) From To Jaljulye Checkpoint, 1 Route 55 The Green Line Partial 3.6 Central east of Qalqiliya West Bank 2 Route 466 Beit El Route 60 Partial 5.5 Giv’at Ze’ev/Neighborhood -

ISRAEL Global Center for Breakthrough
ISRAEL Global Center for Breakthrough www.investinisrael.gov.il Bright Source Dear Reader, Israel is recognized as being Israel’s ability to continuously produce high- at the forefront of high- quality innovative products is fundamental to tech innovation, backed by a its capacity to compete globally and the Israeli highly educated and creative government considers it a high priority to ensure workforce and a sound and support Israel’s future competitiveness. infrastructure. Some of the I am confident that the vitality of the Israeli market world’s largest multinational will continue to position it as a global destination corporations developed their for foreign investment and invite you to take part key breakthroughs in Israel, in our future expansion. and the ongoing influx of new companies serve as a sign of the vibrancy of Israel’s industrial research and development. Israel enjoys the highest percentage in the world Sincerely, of engineers in the workforce and one of the Binyamin (Fouad) Ben Eliezer – highest ratios of university degrees and academic publications per capita. Israel’s finest innovations were developed through the combination of necessity, proven problem solving skills and creativity. Due to the close-knit web of Israeli society, the synergetic nature of Israeli industry Minister of Industry, Trade and Labor allows for cross-sectoral adaptations of technology from the security sector to life sciences, IT and communications. Highly trained graduates of the IDF applied cutting edge defense technology to market-changing civilian applications. 3 Intel Dear Investor, Israeli Innovations Israel has a long track record of An ability to quickly respond to market demands market-creating, profit driving and identify future needs as well as to determine Merging Necessity & Ingenuity Striving for SUCCESS breakthrough innovations and provide solutions to global needs across which have positively impacted different sectors; The technological skill of Israeli life worldwide. -

Arab Citizens' Integration Into Israeli High-Tech
Arab Citizens’ Integration into Israeli High-Tech: Achievements and Emerging Issues Inter-Agency Task Force on Israeli Arab Issues August 2018 _____________________________________ Research: Alma Schneider Editor: Liron Shoham TABLE OF CONTENTS I. INTRODUCTION ............................................................................................................. 1 II. CONTEXT: HIGH TECH SUSTAINABILITY AND GROWTH .................................................... 3 Economic Footprint ................................................................................................................. 3 Human Capital Shortage ......................................................................................................... 4 Focus on Arab Society ............................................................................................................. 5 III. THE CHALLENGE: BARRIERS TO INTEGRATION ................................................................. 6 IV. DOMINANT STRATEGIES AND APPROACHES ................................................................... 8 V. DETAILED STATUS: ACHIEVEMENTS AND EMERGING ISSUES ......................................... 10 Higher Education: Completion and Employability ................................................................ 12 Depth of Workforce Integration ............................................................................................ 13 Industry Expansion – Tech Hubs and Startups ...................................................................... 14 Arab