Use of Gis for Analysis of Community Health Worker Patient
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zambia Page 1 of 8
Zambia Page 1 of 8 Zambia Country Reports on Human Rights Practices - 2003 Released by the Bureau of Democracy, Human Rights, and Labor February 25, 2004 Zambia is a republic governed by a president and a unicameral national assembly. Since 1991, multiparty elections have resulted in the victory of the Movement for Multi-Party Democracy (MMD). MMD candidate Levy Mwanawasa was elected President in 2001, and the MMD won 69 out of 150 elected seats in the National Assembly. Domestic and international observer groups noted general transparency during the voting; however, they criticized several irregularities. Opposition parties challenged the election results in court, and court proceedings were ongoing at year's end. The anti-corruption campaign launched in 2002 continued during the year and resulted in the removal of Vice President Kavindele and the arrest of former President Chiluba and many of his supporters. The Constitution mandates an independent judiciary, and the Government generally respected this provision; however, the judicial system was hampered by lack of resources, inefficiency, and reports of possible corruption. The police, divided into regular and paramilitary units under the Ministry of Home Affairs, have primary responsibility for maintaining law and order. The Zambia Security and Intelligence Service (ZSIS), under the Office of the President, is responsible for intelligence and internal security. Civilian authorities maintained effective control of the security forces. Members of the security forces committed numerous serious human rights abuses. Approximately 60 percent of the labor force worked in agriculture, although agriculture contributed only 15 percent to the gross domestic product. Economic growth increased to 4 percent for the year. -

Zambia Page 1 of 16
Zambia Page 1 of 16 Zambia Country Reports on Human Rights Practices - 2002 Released by the Bureau of Democracy, Human Rights, and Labor March 31, 2003 Zambia is a republic governed by a president and a unicameral national assembly. Since 1991 generally free and fair multiparty elections have resulted in the victory of the Movement for Multi -Party Democracy (MMD). In December 2001, Levy Mwanawasa of the MMD was elected president, and his party won 69 out of 150 elected seats in the National Assembly. The MMD's use of government resources during the campaign raised questions over the fairness of the elections. Although noting general transparency during the voting, domestic and international observer groups cited irregularities in the registration process and problems in the tabulation of the election results. Opposition parties challenged the election result in court, and court proceedings remained ongoing at year's end. The Constitution mandates an independent judiciary, and the Government generally respected this provision; however, the judicial system was hampered by lack of resources, inefficiency, and reports of possible corruption. The police, divided into regular and paramilitary units operated under the Ministry of Home Affairs, had primary responsibility for maintaining law and order. The Zambia Security and Intelligence Service (ZSIS), under the Office of the President, was responsible for intelligence and internal security. Members of the security forces committed numerous, and at times serious, human rights abuses. Approximately 60 percent of the labor force worked in agriculture, although agriculture contributed only 22 percent to the gross domestic product. Economic growth slowed to 3 percent for the year, partly as a result of drought in some agricultural areas. -

Effectiveness of Community Participation in Decision
EFFECTIVENESS OF COMMUNITY PARTICIPATION IN DECISION MAKING AND IMPLEMENTATION OF WATER SUPPLY AND SANITATION PROJECTS: A CASE STUDY OF CHONGWE DISTRICT BY TIMOTHY PHIRI A DISSERTATION SUBMITTED TO THE UNIVERSITY OF ZAMBIA IN PARTIAL FULFILMENT OF THE REQUIREMENT OF THE DEGREE OF MASTER OF PUBLIC ADMINISTRATION (MPA) THE UNIVERSITY OF ZAMBIA LUSAKA 2015 DECLARATION I, TIMOTHY PHIRI, declare that this dissertation represents my own work and that it has not been previously submitted for a degree, diploma or other qualifications at this or another university. Signature: ___________________________________________ Date: _______________________________________________ i CERTIFICATE OF APPROVAL FORM This dissertation of TIMOTHY PHIRI has been approved as partial fulfilment of the requirement for the award of the Degree of Master of Public Administration by the University of Zambia. Examiner Signature Date __________________ __________________ _________ __________________ ___________________ _________ __________________ ____________________ _________ ii COPYRIGHT All rights reserved. No part of this dissertation may be reproduced or stored in any form or by any means without prior permission in writing from the author. iii DEDICATION To Agness Falesi Phiri iv ABSTRACT The study investigated factors that lead to low levels of community participation in decision making and implementation of water supply and sanitation projects in Chongwe District. Focus was on five political units (wards) namely Nthandabale, Chalimbana, Nakatindi, Lukoshi and Chongwe. -
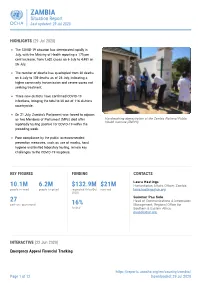
ZAMBIA Situation Report Last Updated: 29 Jul 2020
ZAMBIA Situation Report Last updated: 29 Jul 2020 HIGHLIGHTS (29 Jul 2020) The COVID-19 situation has deteriorated rapidly in July, with the Ministry of Health reporting a 175 per cent increase, from 1,632 cases on 6 July to 4,481 on 26 July. The number of deaths has quadrupled from 30 deaths on 6 July to 139 deaths as of 26 July, indicating a higher community transmission and severe cases not seeking treatment. Three new districts have confirmed COVID-19 infections, bringing the total to 38 out of 116 districts countrywide. On 21 July, Zambia’s Parliament was forced to adjourn as two Members of Parliament (MPs) died after Handwashing demostration at the Zambia National Public Health Institute (ZNPHI) reportedly testing positive for COVID-19 within the preceding week. Poor compliance by the public to recommended prevention measures, such as use of masks, hand hygiene and limited laboratory testing, remain key challenges to the COVID-19 response. KEY FIGURES FUNDING CONTACTS Laura Hastings 10.1M 6.2M $132.9M $21M Humanitarian Affairs Officer, Zambia people in need people targeted requested (May-Oct received [email protected] 2020) Guiomar Pau Sole 27 Head of Communications & Information partners operational 16% Management, Regional Office for funded Southern & Eastern Africa [email protected] INTERACTIVE (22 Jun 2020) Emergency Appeal Financial Tracking https://reports.unocha.org/en/country/zambia/ Page 1 of 12 Downloaded: 29 Jul 2020 ZAMBIA Situation Report Last updated: 29 Jul 2020 View this interactive graphic: https://bit.ly/ZambiaAppeal2020Funding BACKGROUND (29 Jul 2020) Situation Overview Zambia recorded the first case of COVID-19 on 18 March 2020, and as 26 July 4,481 cases had been confirmed with 139 deaths reported, in 38 out of 116 districts countrywide, according to the Ministry of Health (MoH). -
C:\Users\Public\Documents\GP JOBS\Gazette No. 73 of Friday, 16Th
REPUBLIC OF ZAMBIA Price: K5 net Annual Subscription: Within Lusaka—K200.00 Published by Authority Outside Lusaka—K230.00 No. 6430] Lusaka, Friday, 16th October, 2015 [Vol. LI, No. 73 GAZETTE NOTICE NO. 643 OF 2015 [5929855/13 Zambia Information and Communications Technologies Authority The Information and Communications Technologies Act, 2009 (Act No. 15 of 2009) Notice of Determination of Unserved and Underserved Areas Section 70 (2) of the Information and Communication TechnologiesAct No. 15 of 2009 (ICTAct) empowers the Zambia Information and Communications Technology Authority (ZICTA) to determine a system to promote the widespread availability and usage of electronic communications networks and services throughout Zambia by encouraging the installation of electronic communications networks and the provision for electronic communications services in unserved and underserved areas and communities. Further, Regulation 5 (2) of Statutory Instrument No. 38 of 2012 the Information and Communications Technologies (Universal Access) Regulations 2012 mandates the Authority to designate areas as universal service areas by notice in the gazette. In accordance with the said regulations, the Authority hereby notifies members of the public that areas contained in the Schedule Hereto are hereby designated as universal service areas. M. K. C. MUDENDA (MRS.) Director General SN Site Name Longtitude Latitude Elevation Province 1 Nalusanga_Chunga Headquarter Offices 27.22415 -15.22135 1162 Central 2 Mpusu_KankamoHill 27.03507 -14.45675 1206 Central -

Observing the 2001 Zambia Elections
SPECIAL REPORT SERIES THE CARTER CENTER WAGING PEACE ◆ FIGHTING DISEASE ◆ BUILDING HOPE OBSERVING THE 2001 ZAMBIA ELECTIONS THE CARTER CENTER STRIVES TO RELIEVE SUFFERING BY ADVANCING PEACE AND HEALTH WORLDWIDE; IT SEEKS TO PREVENT AND RESOLVE CONFLICTS, ENHANCE FREEDOM AND DEMOCRACY, AND PROTECT AND PROMOTE HUMAN RIGHTS WORLDWIDE. THE CARTER CENTER NDINDI OBSERVING THE 2001 ZAMBIA ELECTIONS OBSERVING THE 2001 ZAMBIA ELECTIONS FINAL REPORT THE CARTER CENTER The Democracy Program One Copenhill Atlanta, GA 30307 (404) 420-5188 FAX (404) 420-5196 WWW.CARTERCENTER.ORG OCTOBER 2002 1 THE CARTER CENTER NDI OBSERVING THE 2001 ZAMBIA ELECTIONS 2 THE CARTER CENTER NDINDI OBSERVING THE 2001 ZAMBIA ELECTIONS TABLE OF CONTENTS Carter Center Election Observation Delegation and Staff ............................................................... 5 Terms and Abbreviations ................................................................................................................. 7 Foreword ......................................................................................................................................... 8 Executive Summary .......................................................................................................................... 10 Acknowledgments............................................................................................................................. 15 Background ...................................................................................................................................... -

Report of the Parliamentary Reforms and Modernisation Committee for the Third Session of the Eleventh National Assembly Appointed on 24Th September, 2014
REPORT OF THE PARLIAMENTARY REFORMS AND MODERNISATION COMMITTEE FOR THE THIRD SESSION OF THE ELEVENTH NATIONAL ASSEMBLY APPOINTED ON 24TH SEPTEMBER, 2014 Consisting of: Mr S Katuka, MP, (Chairperson); Hon A Chikwanda, MP, Minister of Finance; Hon W M Kabimba, SC, MP, Minister of Justice; Hon E Kabanshi, MP, Minister of Local Government and Housing, Hon C K Banda, SC, MP; Deputy Chairperson of Committees of the Whole House, Ms M Lubezhi, MP; Mr P Mucheleka, MP; MR L C Bwalya, MP; Mr L J Ngoma, MP; and Mr I K Banda, MP. The composition of your Committee changed in the course of the session when Hon E Kabanshi, Minister of Local Government and Housing then was appointed to the Ministry of Community Development, Mother and Child Health. She was subsequently replaced by Hon E Chenda, Minister of Commerce then who was appointed as the new Minister of Local Government and Housing. The Honourable Mr Speaker National Assembly Parliament Building LUSAKA Sir, Your Committee has the honour to present its report for the Third Session of the Eleventh National Assembly. 2. Functions of the Committee Your Committee was guided in all its deliberations by Standing Order No. 152 which set out the functions of your Committee as set out below. 1 (i) In addition to any other work placed upon it by any Standing Orders of the Assembly, it shall be the duty of the Committee to examine and propose reform to the powers, procedures and practices, organisation and facilities of the Assembly, provided that in proposing such reforms, the Committee shall bear in mind the balance of power between the respective constitutional responsibilities, roles of the National Assembly and the Government and the duties of other House Keeping Committees. -

Registered Voters by Gender and Constituency
REGISTERED VOTERS BY GENDER AND CONSTITUENCY % OF % OF SUB % OF PROVINCIAL CONSTITUENCY NAME MALES MALES FEMALES FEMALES TOTAL TOTAL KATUBA 25,040 46.6% 28,746 53.4% 53,786 8.1% KEEMBE 23,580 48.1% 25,453 51.9% 49,033 7.4% CHISAMBA 19,289 47.5% 21,343 52.5% 40,632 6.1% CHITAMBO 11,720 44.1% 14,879 55.9% 26,599 4.0% ITEZH-ITEZHI 18,713 47.2% 20,928 52.8% 39,641 5.9% BWACHA 24,749 48.1% 26,707 51.9% 51,456 7.7% KABWE CENTRAL 31,504 47.4% 34,993 52.6% 66,497 10.0% KAPIRI MPOSHI 41,947 46.7% 47,905 53.3% 89,852 13.5% MKUSHI SOUTH 10,797 47.3% 12,017 52.7% 22,814 3.4% MKUSHI NORTH 26,983 49.5% 27,504 50.5% 54,487 8.2% MUMBWA 23,494 47.9% 25,545 52.1% 49,039 7.4% NANGOMA 12,487 47.4% 13,864 52.6% 26,351 4.0% LUFUBU 5,491 48.1% 5,920 51.9% 11,411 1.7% MUCHINGA 10,072 49.7% 10,200 50.3% 20,272 3.0% SERENJE 14,415 48.5% 15,313 51.5% 29,728 4.5% MWEMBEZHI 16,756 47.9% 18,246 52.1% 35,002 5.3% 317,037 47.6% 349,563 52.4% 666,600 100.0% % OF % OF SUB % OF PROVINCIAL CONSTITUENCY NAME MALES MALES FEMALES FEMALES TOTAL TOTAL CHILILABOMBWE 28,058 51.1% 26,835 48.9% 54,893 5.4% CHINGOLA 34,695 49.7% 35,098 50.3% 69,793 6.8% NCHANGA 23,622 50.0% 23,654 50.0% 47,276 4.6% KALULUSHI 32,683 50.1% 32,614 49.9% 65,297 6.4% CHIMWEMWE 29,370 48.7% 30,953 51.3% 60,323 5.9% KAMFINSA 24,282 51.1% 23,214 48.9% 47,496 4.6% KWACHA 31,637 49.3% 32,508 50.7% 64,145 6.3% NKANA 27,595 51.9% 25,562 48.1% 53,157 5.2% WUSAKILE 23,206 50.5% 22,787 49.5% 45,993 4.5% LUANSHYA 26,658 49.5% 27,225 50.5% 53,883 5.3% ROAN 15,921 50.1% 15,880 49.9% 31,801 3.1% LUFWANYAMA 18,023 50.2% -

Members of the Northern Rhodesia Legislative Council and National Assembly of Zambia, 1924-2021
NATIONAL ASSEMBLY OF ZAMBIA Parliament Buildings P.O Box 31299 Lusaka www.parliament.gov.zm MEMBERS OF THE NORTHERN RHODESIA LEGISLATIVE COUNCIL AND NATIONAL ASSEMBLY OF ZAMBIA, 1924-2021 FIRST EDITION, 2021 TABLE OF CONTENTS FOREWORD ................................................................................................................................................ 3 PREFACE ..................................................................................................................................................... 4 ACKNOWLEDGEMENTS .......................................................................................................................... 5 ABBREVIATIONS ...................................................................................................................................... 7 INTRODUCTION ........................................................................................................................................ 9 PART A: MEMBERS OF THE LEGISLATIVE COUNCIL, 1924 - 1964 ............................................... 10 PRIME MINISTERS OF THE FEDERATION OF RHODESIA .......................................................... 12 GOVERNORS OF NORTHERN RHODESIA AND PRESIDING OFFICERS OF THE LEGISTRATIVE COUNCIL (LEGICO) ............................................................................................... 13 SPEAKERS OF THE LEGISTRATIVE COUNCIL (LEGICO) - 1948 TO 1964 ................................. 16 DEPUTY SPEAKERS OF THE LEGICO 1948 TO 1964 .................................................................... -

5 the CDF in Zambia: Governing Rules and Implementation 56 5.1 an Overview 56
DIPLOMARBEIT Titel der Diplomarbeit Governance of the Constituency Development Fund in Zambia Verfasser Matthias Zingel, BA angestrebter akademischer Grad Magister (Mag.) Wien, Oktober 2012 Studienkennzahl lt. Studienblatt: A 057 390 Studienrichtung lt. Studienblatt: Internationale Entwicklung Betreuer: Univ. Prof. Dr. Walter Schicho i Table of Figures v Acknowledgment vii List of Abbrevations viii 1 Introduction 1 2 Methodology 3 2.1 The Dilemma of Planning & Conducting a Field Research 3 2.2 Limitations of the study 4 2.3 Place and Quality of Research 5 2.4 Research Agenda 6 2.5 Research Design 7 2.6 Data Collection 7 2.6.1 Structured Sampling for Validity 7 2.6.2 Semi-Structured Interviews 8 2.7 Data Analysis 9 2.7.1 Transcript of Interviews 9 2.7.2 Content Analysis 9 3 Decentralization and Local Governance 11 3.1 Decentralization 11 3.1.1 Terminological Issues 11 3.1.2 Typologies 12 3.1.2.1 Deconcentration 12 3.1.2.2 Fiscal decentralization 13 3.1.2.3 Devolution 14 3.1.3 Purposes of Decentralization 15 3.1.3.1 Administration purpose 15 3.1.3.2 Economic Purposes 16 3.1.3.3 Political Purposes 17 3.1.4 Pitfalls of Decentralization 18 3.1.4.1 Initiators of Decentralization 19 3.1.4.2 The Problem of Opportunistic Local Elites 19 3.1.4.3 The Problem of Adequate Resources 20 3.2 Local Governance 23 3.2.1 Transparency 23 ii 3.2.1.1 Transparency of decision making 26 3.1.2.2 The linkage between transparency and accountability 26 3.2.2 Participation 28 3.2.2.1 Social and Project Participation 28 3.2.2.2 Political Participation 29 3.2.2.3 Participatory -

Chiefdoms/Chiefs in Zambia
CHIEFDOMS/CHIEFS IN ZAMBIA 1. CENTRAL PROVINCE A. Chibombo District Tribe 1 HRH Chief Chitanda Lenje People 2 HRH Chieftainess Mungule Lenje People 3 HRH Chief Liteta Lenje People B. Chisamba District 1 HRH Chief Chamuka Lenje People C. Kapiri Mposhi District 1 HRH Senior Chief Chipepo Lenje People 2 HRH Chief Mukonchi Swaka People 3 HRH Chief Nkole Swaka People D. Ngabwe District 1 HRH Chief Ngabwe Lima/Lenje People 2 HRH Chief Mukubwe Lima/Lenje People E. Mkushi District 1 HRHChief Chitina Swaka People 2 HRH Chief Shaibila Lala People 3 HRH Chief Mulungwe Lala People F. Luano District 1 HRH Senior Chief Mboroma Lala People 2 HRH Chief Chembe Lala People 3 HRH Chief Chikupili Swaka People 4 HRH Chief Kanyesha Lala People 5 HRHChief Kaundula Lala People 6 HRH Chief Mboshya Lala People G. Mumbwa District 1 HRH Chief Chibuluma Kaonde/Ila People 2 HRH Chieftainess Kabulwebulwe Nkoya People 3 HRH Chief Kaindu Kaonde People 4 HRH Chief Moono Ila People 5 HRH Chief Mulendema Ila People 6 HRH Chief Mumba Kaonde People H. Serenje District 1 HRH Senior Chief Muchinda Lala People 2 HRH Chief Kabamba Lala People 3 HRh Chief Chisomo Lala People 4 HRH Chief Mailo Lala People 5 HRH Chieftainess Serenje Lala People 6 HRH Chief Chibale Lala People I. Chitambo District 1 HRH Chief Chitambo Lala People 2 HRH Chief Muchinka Lala People J. Itezhi Tezhi District 1 HRH Chieftainess Muwezwa Ila People 2 HRH Chief Chilyabufu Ila People 3 HRH Chief Musungwa Ila People 4 HRH Chief Shezongo Ila People 5 HRH Chief Shimbizhi Ila People 6 HRH Chief Kaingu Ila People K. -

PDO Indicator: Number of Students Enrolled in Grades 8–12 in the Expanded Secondary Schools with Additional Classrooms and Facilities
ZAMBIA EDUCATION ENHANCEMENT PROJECT INDICATOR FRAMEWORK REPORT – 25/11/2019 PDO Indicator: Number of students enrolled in Grades 8–12 in the expanded secondary schools with additional classrooms and facilities. (Number) Component Indicator YR1 (July 2018 Baseline YR2 (July2019-June2020) type June2019) Indicator Name Target Actual Target Actual Number of students enrolled in Grades 8–12 in PDO the expanded secondary schools with additional 0 0 0 4,920 6,364 Indicators 2 classrooms and facilities. (Number) • A total of 6,364 learners were enrolled in the 82 expansion schools 1 PDO Indicators: Student Benefiting from Direct Intervention Comp Indicator YR1 (July 2018 YR2 (July2019- onent Baseline type June2019) June2020) Indicator Name Target Actual Target Actual PDO Students benefiting from direct interventions to 0 0 0 146,920 Indicators enhance learning (Number) - (Corporate) 76,400 ALL Students benefiting from direct interventions to PDO enhance learning - Female (Number - Sub-Type: 0 0 0 73,778 Indicators 23,470 Supplemental) - (Corporate) ALL • There are a total of 146,920 students benefiting from direct interventions to enhance learning. This number is derived from the actual enrolments received from 251 out of 382 Pilot schools as shown in Table 2 • The data has been compiled from real time submissions by schools. 2 Table 2: Enrolments of learners benefiting from direct interventions Enrolments Primary Secondary Total Sn Province District School B G T B G T B G T 1 Central Chisamba Chabusha Primary 219 248 467 48 30 78 267 278 545