Response Properties and Biological Function of the Skate Electrosensory System During Ontogeny
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Age, Growth, and Sexual Maturity of the Deepsea Skate, Bathyraja
AGE, GROWTH, AND SEXUAL MATURITY OF THE DEEPSEA SKATE, BATHYRAJA ABYSSICOLA A Thesis Presented to the Faculty of Alaska Pacific University In Partial Fulfillment of the Requirements For the Degree of Master of Science in Environmental Science by Cameron Murray Provost April 2016 Pro Q u est Nu m b er: 10104548 All rig hts reserv e d INF O RM ATI O N T O ALL USERS Th e q u a lity of this re pro d u ctio n is d e p e n d e nt u p o n th e q u a lity of th e c o p y su b mitt e d. In th e unlik e ly e v e nt th a t th e a uth or did n ot se n d a c o m ple t e m a nuscript a n d th ere are missin g p a g es, th ese will b e n ot e d. Also, if m a t eria l h a d to b e re m o v e d, a n ot e will in dic a t e th e d e le tio n. Pro Q u est 10104548 Pu blish e d b y Pro Q u est LL C (2016). C o p yrig ht of th e Dissert a tio n is h e ld b y th e A uth or. All rig hts reserv e d. This w ork is prot e ct e d a g a inst un a uth orize d c o p yin g un d er Title 17, Unit e d St a t es C o d e Microform Editio n © Pro Q u est LL C . -

Barndoor Skate, Dipturus Laevis, Life History and Habitat Characteristics
NOAA Technical Memorandum NMFS-NE-173 Essential Fish Habitat Source Document: Barndoor Skate, Dipturus laevis, Life History and Habitat Characteristics U. S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Northeast Region Northeast Fisheries Science Center Woods Hole, Massachusetts March 2003 Recent Issues in This Series: 155. Food of Northwest Atlantic Fishes and Two Common Species of Squid. By Ray E. Bowman, Charles E. Stillwell, William L. Michaels, and Marvin D. Grosslein. January 2000. xiv + 138 p., 1 fig., 7 tables, 2 app. NTIS Access. No. PB2000-106735. 156. Proceedings of the Summer Flounder Aging Workshop, 1-2 February 1999, Woods Hole, Massachusetts. By George R. Bolz, James Patrick Monaghan, Jr., Kathy L. Lang, Randall W. Gregory, and Jay M. Burnett. May 2000. v + 15 p., 5 figs., 5 tables. NTIS Access. No. PB2000-107403. 157. Contaminant Levels in Muscle of Four Species of Recreational Fish from the New York Bight Apex. By Ashok D. Deshpande, Andrew F.J. Draxler, Vincent S. Zdanowicz, Mary E. Schrock, Anthony J. Paulson, Thomas W. Finneran, Beth L. Sharack, Kathy Corbo, Linda Arlen, Elizabeth A. Leimburg, Bruce W. Dockum, Robert A. Pikanowski, Brian May, and Lisa B. Rosman. June 2000. xxii + 99 p., 6 figs., 80 tables, 3 app., glossary. NTIS Access. No. PB2001-107346. 158. A Framework for Monitoring and Assessing Socioeconomics and Governance of Large Marine Ecosystems. By Jon G. Sutinen, editor, with contributors (listed alphabetically) Patricia Clay, Christopher L. Dyer, Steven F. Edwards, John Gates, Tom A. Grigalunas, Timothy Hennessey, Lawrence Juda, Andrew W. Kitts, Philip N. -

Chondrichthyan Fishes (Sharks, Skates, Rays) Announcements
Chondrichthyan Fishes (sharks, skates, rays) Announcements 1. Please review the syllabus for reading and lab information! 2. Please do the readings: for this week posted now. 3. Lab sections: 4. i) Dylan Wainwright, Thursday 2 - 4/5 pm ii) Kelsey Lucas, Friday 2 - 4/5 pm iii) Labs are in the Northwest Building basement (room B141) 4. Lab sections done: first lab this week on Thursday! 5. First lab reading: Agassiz fish story; lab will be a bit shorter 6. Office hours: we’ll set these later this week Please use the course web site: note the various modules Outline Lecture outline: -- Intro. to chondrichthyan phylogeny -- 6 key chondrichthyan defining traits (synapomorphies) -- 3 chondrichthyan behaviors -- Focus on several major groups and selected especially interesting ones 1) Holocephalans (chimaeras or ratfishes) 2) Elasmobranchii (sharks, skates, rays) 3) Batoids (skates, rays, and sawfish) 4) Sharks – several interesting groups Not remotely possible to discuss today all the interesting groups! Vertebrate tree – key ―fish‖ groups Today Chondrichthyan Fishes sharks Overview: 1. Mostly marine 2. ~ 1,200 species 518 species of sharks 650 species of rays 38 species of chimaeras Skates and rays 3. ~ 3 % of all ―fishes‖ 4. Internal skeleton made of cartilage 5. Three major groups 6. Tremendous diversity of behavior and structure and function Chimaeras Chondrichthyan Fishes: 6 key traits Synapomorphy 1: dentition; tooth replacement pattern • Teeth are not fused to jaws • New rows move up to replace old/lost teeth • Chondrichthyan teeth are -

Skates and Rays Diversity, Exploration and Conservation – Case-Study of the Thornback Ray, Raja Clavata
UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Doutoramento em Ciências do Mar 2010 UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA ANIMAL SKATES AND RAYS DIVERSITY, EXPLORATION AND CONSERVATION – CASE-STUDY OF THE THORNBACK RAY, RAJA CLAVATA Bárbara Marques Serra Pereira Tese orientada por Professor Auxiliar com Agregação Leonel Serrano Gordo e Investigadora Auxiliar Ivone Figueiredo Doutoramento em Ciências do Mar 2010 The research reported in this thesis was carried out at the Instituto de Investigação das Pescas e do Mar (IPIMAR - INRB), Unidade de Recursos Marinhos e Sustentabilidade. This research was funded by Fundação para a Ciência e a Tecnologia (FCT) through a PhD grant (SFRH/BD/23777/2005) and the research project EU Data Collection/DCR (PNAB). Skates and rays diversity, exploration and conservation | Table of Contents Table of Contents List of Figures ............................................................................................................................. i List of Tables ............................................................................................................................. v List of Abbreviations ............................................................................................................. viii Agradecimentos ........................................................................................................................ -

An Introduction to the Classification of Elasmobranchs
An introduction to the classification of elasmobranchs 17 Rekha J. Nair and P.U Zacharia Central Marine Fisheries Research Institute, Kochi-682 018 Introduction eyed, stomachless, deep-sea creatures that possess an upper jaw which is fused to its cranium (unlike in sharks). The term Elasmobranchs or chondrichthyans refers to the The great majority of the commercially important species of group of marine organisms with a skeleton made of cartilage. chondrichthyans are elasmobranchs. The latter are named They include sharks, skates, rays and chimaeras. These for their plated gills which communicate to the exterior by organisms are characterised by and differ from their sister 5–7 openings. In total, there are about 869+ extant species group of bony fishes in the characteristics like cartilaginous of elasmobranchs, with about 400+ of those being sharks skeleton, absence of swim bladders and presence of five and the rest skates and rays. Taxonomy is also perhaps to seven pairs of naked gill slits that are not covered by an infamously known for its constant, yet essential, revisions operculum. The chondrichthyans which are placed in Class of the relationships and identity of different organisms. Elasmobranchii are grouped into two main subdivisions Classification of elasmobranchs certainly does not evade this Holocephalii (Chimaeras or ratfishes and elephant fishes) process, and species are sometimes lumped in with other with three families and approximately 37 species inhabiting species, or renamed, or assigned to different families and deep cool waters; and the Elasmobranchii, which is a large, other taxonomic groupings. It is certain, however, that such diverse group (sharks, skates and rays) with representatives revisions will clarify our view of the taxonomy and phylogeny in all types of environments, from fresh waters to the bottom (evolutionary relationships) of elasmobranchs, leading to a of marine trenches and from polar regions to warm tropical better understanding of how these creatures evolved. -

Sharks and Rays Back in the North Sea!
SHARKS AND RAYS BACK IN THE NORTH SEA! © Peter Verhoog Juvenile thornback rays ABOUT SHARKS AND RAYS Important for the marine ecosystem Long before we humans walked this earth, beautiful sharks and rays were swimming in the world’s oceans. These apex predators have been at the top of the food chain for 450 million years, where they fulfil a vital role in the ecosystem of the oceans. They ‘maintain’ coral reefs, they ensure the ecological balance between species and they contribute to healthy populations of other animals by eating the weaker individuals. Largescale overfishing of sharks and rays affects the entire food chain and thus the health of the oceans. Low reproductive capacity Sharks and rays are characterized by a low reproductive capacity; many species are only sexually mature after more than 10 years. Most shark species develop their eggs inside the body of the female. Baby sharks are born fully developed. Most sharks have 1 to 20 pups per year. The reproductive biology of sharks is therefore more akin to that of marine mammals than fish. The Dutch North Sea is the habitat of several egg-laying shark species and rays: both the small-spotted catshark and the greater spotted dogfish or nursehound lay 18 to 20 eggs per year. The eggs take 7 to 10 months to hatch. The empty cases often wash up on beaches. Most Dutch bottom-dwelling rays lay eggs, from 20 to 140 eggs per year. Due to their low reproduction cycles, sharks and rays are vulnerable for overfishing, as the recovery of populations is very slow. -

(Chondrichthyes : Rajidae), Okamejei Mengae from the South China Sea
Korean J. Ichthyol. 19(1), 57~65, 2007 A New Species of Skate (Chondrichthyes : Rajidae), Okamejei mengae from the South China Sea Choong-Hoon Jeong*, Tetsuji Nakabo1 and Han-Ling Wu2 Research Center for Coastal Environments of Yellow Sea, Inha University, Incheon 402-751, Republic of Korea 1The Kyoto University Museum, c/o Division of Applied Biosciences, Kyoto University, Kyoto 606-8501, Japan 2Laboratory of Fishes, Shanghai Fisheries University, 334 Jun Gong Rd., Shanghai, 200090, People’s of Republic of China A new species of the rajid genus Okamejei is described from a single specimen (295 mm TL) from off Shantou, Gwangdong in the South China Sea. The new species differs from all other congeners in the following combination of characters: snout pointed, dorsal head length 6.7 times interorbital width, tail moderately wide and long, its length 48.5% TL, interdorsal distance less than length of first dorsal fin base, postdorsal tail short as 5.8% TL, small evenly distributed dark brownish spots, without ocelli on dorsal surface of disc, pores of ampullae of Lorenzini on ventral surface distributed from snout tip to distal end of metapterygium, scapulocoracoid high, its height about 1.4 times rear corner height, trunk vertebrae 23, predorsal tail vertebrae 50 and pectoral fin radials 96. Key words : New species, Okamejei mengae, Rajidae, South China Sea, Chondri- chthyes size and condition of the anterior margin of the Introduction neurocranial fontanelle. Okamejei was later ele- vated to generic rank by McEachran and Dunn The family Rajidae is cosmopolitan, encompass- (1998). To date, genus Okamejei comprised nine ing about thirty genera and more than 230 nomi- species, three distributed in the Indian Ocean nal species, as well as about 50 undescribed spe- (Stehmann, 1976; Fricke and Al-Hassan, 1995) cies (McEachran and Miyake, 1990a, b; McEach- and six in the western North Pacific (reviewed by ran and Dunn, 1998). -

Florida's Fintastic Sharks and Rays Lesson and Activity Packet
Florida's Fintastic Sharks and Rays An at-home lesson for grades 3-5 Produced by: This educational workbook was produced through the support of the Indian River Lagoon National Estuary Program. 1 What are sharks and rays? Believe it or not, they’re a type of fish! When you think “fish,” you probably picture a trout or tuna, but fishes come in all shapes and sizes. All fishes share the following key characteristics that classify them into this group: Fishes have the simplest of vertebrate hearts with only two chambers- one atrium and one ventricle. The spine in a fish runs down the middle of its back just like ours, making fish vertebrates. All fishes have skeletons, but not all fish skeletons are made out of bones. Some fishes have skeletons made out of cartilage, just like your nose and ears. Fishes are cold-blooded. Cold-blooded animals use their environment to warm up or cool down. Fins help fish swim. Fins come in pairs, like pectoral and pelvic fins or are singular, like caudal or anal fins. Later in this packet, we will look at the different types of fins that fishes have and some of the unique ways they are used. 2 Placoid Ctenoid Ganoid Cycloid Hard protective scales cover the skin of many fish species. Scales can act as “fingerprints” to help identify some fish species. There are several different scale types found in bony fishes, including cycloid (round), ganoid (rectangular or diamond), and ctenoid (scalloped). Cartilaginous fishes have dermal denticles (Placoid) that resemble tiny teeth on their skin. -

Leucoraja Erinacea
Little Skate − Leucoraja erinacea Overall Vulnerability Rank = Low Biological Sensitivity = Low Climate Exposure = High Data Quality = 88% of scores ≥ 2 Expert Data Expert Scores Plots Leucoraja erinacea Scores Quality (Portion by Category) Low Moderate Stock Status 2.0 2.8 High Other Stressors 1.5 1.4 Very High Population Growth Rate 2.9 2.4 Spawning Cycle 1.2 3.0 Complexity in Reproduction 1.3 2.2 Early Life History Requirements 1.1 3.0 Sensitivity to Ocean Acidification 1.5 2.8 Prey Specialization 1.2 3.0 Habitat Specialization 1.2 3.0 Sensitivity attributes Sensitivity to Temperature 2.1 3.0 Adult Mobility 2.3 2.2 Dispersal & Early Life History 1.9 2.8 Sensitivity Score Low Sea Surface Temperature 3.9 3.0 Variability in Sea Surface Temperature 1.0 3.0 Salinity 2.0 3.0 Variability Salinity 1.2 3.0 Air Temperature 1.0 3.0 Variability Air Temperature 1.0 3.0 Precipitation 1.0 3.0 Variability in Precipitation 1.0 3.0 Ocean Acidification 4.0 2.0 Exposure variables Variability in Ocean Acidification 1.0 2.2 Currents 2.1 1.0 Sea Level Rise 1.1 1.5 Exposure Score High Overall Vulnerability Rank Low Little Skate (Leucoraja erinacea) Overall Climate Vulnerability Rank: Low (88% certainty from bootstrap analysis). Climate Exposure: High. Two exposure factors contributed to this score: Ocean Surface Temperature (3.9) and Ocean Acidification (4.0). Little Skate are demersal and complete their life cycle in marine habitats. Biological Sensitivity: Low. Only one attribute scored above 2.5: Population Growth Rate (3.4). -

Class Wars: Chondrichthyes and Osteichthyes Dominance in Chesapeake Bay, 2002-2012
Class Wars: Chondrichthyes and Osteichthyes dominance in Chesapeake Bay, 2002-2012. 01 July 2013 Introduction The objective of this analysis was to demonstrate a possible changing relationship between two Classes of fishes, Osteichthyes (the bony fishes) and Chondrichthyes (the cartilaginous fishes) in Chesapeake Bay based on 11 years of monitoring. If any changes between the two Classes appeared to be significant, either statistically or anecdotally, the data were explored further in an attempt to explain the variation. The Class Osteichthyes is characterized by having a skeleton made of bone and is comprised of the majority of fish species worldwide, while the Chondrichthyes skeleton is made of cartilage and is represented by the sharks, skates, and rays (the elasmobranch fishes) and chimaeras1. Many shark species are generally categorized as apex predators, while skates and rays and some smaller sharks can be placed into the mesopredator functional group (Myers et al., 2007). By definition, mesopredators prey upon a significant array of lower trophic groups, but also serve as the prey base for apex predators. Global demand for shark and consequential shark fishing mortality, estimated at 97 million sharks in 2010 (Worm et al., 2013), is hypothesized to have contributed to the decline of these apex predators in recent years (Baum et al., 2003 and Fowler et al., 2005), which in turn is suggested to have had a cascading effect on lower trophic levels—an increase in mesopredators and subsequent decrease in the prey base (Myers et al., 2007). According to 10 years of trawl survey monitoring of Chesapeake Bay, fish species composition of catches has shown a marked change over the years (Buchheister et al., 2013). -

An Annotated Checklist of the Chondrichthyan Fishes Inhabiting the Northern Gulf of Mexico Part 1: Batoidea
Zootaxa 4803 (2): 281–315 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2020 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4803.2.3 http://zoobank.org/urn:lsid:zoobank.org:pub:325DB7EF-94F7-4726-BC18-7B074D3CB886 An annotated checklist of the chondrichthyan fishes inhabiting the northern Gulf of Mexico Part 1: Batoidea CHRISTIAN M. JONES1,*, WILLIAM B. DRIGGERS III1,4, KRISTIN M. HANNAN2, ERIC R. HOFFMAYER1,5, LISA M. JONES1,6 & SANDRA J. RAREDON3 1National Marine Fisheries Service, Southeast Fisheries Science Center, Mississippi Laboratories, 3209 Frederic Street, Pascagoula, Mississippi, U.S.A. 2Riverside Technologies Inc., Southeast Fisheries Science Center, Mississippi Laboratories, 3209 Frederic Street, Pascagoula, Missis- sippi, U.S.A. �[email protected]; https://orcid.org/0000-0002-2687-3331 3Smithsonian Institution, Division of Fishes, Museum Support Center, 4210 Silver Hill Road, Suitland, Maryland, U.S.A. �[email protected]; https://orcid.org/0000-0002-8295-6000 4 �[email protected]; https://orcid.org/0000-0001-8577-968X 5 �[email protected]; https://orcid.org/0000-0001-5297-9546 6 �[email protected]; https://orcid.org/0000-0003-2228-7156 *Corresponding author. �[email protected]; https://orcid.org/0000-0001-5093-1127 Abstract Herein we consolidate the information available concerning the biodiversity of batoid fishes in the northern Gulf of Mexico, including nearly 70 years of survey data collected by the National Marine Fisheries Service, Mississippi Laboratories and their predecessors. We document 41 species proposed to occur in the northern Gulf of Mexico. -
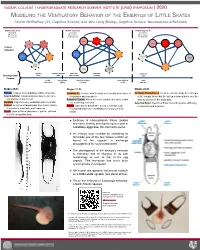
Modeling the Ventilatory Behavior of the Embryos Of
VASSAR COLLEGE | UNDERGRADUATE RESEARCH SUMMER INSTITUTE (URSI) SYMPOSIUM | 2020 MODELING THE VENTILATORY BEHAVIOR OF THE EMBRYOS OF LITTLE SKATES Connor McShaffrey (‘21, Cognitive Science) and John Long (Biology, Cognitive Science, Neuroscience & Behavior) retract freeze recoil retract freeze recoil retract recoil ventilate pulsate (L) select & pulsate ventilate return to enter tendril search ventilate & enter front- search return to faced & enter tendril Stages 30-31 Stages 31-32 Stages 32-33 Pulsate: Gradual, slow undulation without insertion. Ventilate (L): Ventilate with the body bent laterally at the base of Ventilate Front-Faced: Ventilate with the body bent sharply Search & Enter: Rapid undulation down to tail tip to the putative adult caudal fin. at the caudal fin so that the tail tip is looped back over the find and insert into a tendril. Return to Tendril: After a full recoil, return to the same tendril anterior portion of the body disk.. Ventilate: High frequency undulation within a tendril. that was being ventilated. Select & Enter: Search & Enter, but with greater efficiency Retract : An incremental pull-back from tendril relative Freeze: Upon partial disturbance during ventilation, stop of selection and insertion. to sensitivity, amplitude, and frequency. moving and wait before ventilating or moving out of the Recoil: Upon sufficient disturbance, pull the tail back tendril. to coil it around the body. ● Embryos of elasmobranch fishes (skates and some sharks) develop for up to a year in a leathery egg case, the mermaid’s purse. ● An embryo must ventilate by undulating its tail inside one of the four hollow tendrils (or horns) of the capsule to exchange deoxygenated for oxygenated water.