Evaluating the Potential for Grey Seal Predation to Explain Elevated Natural Mortality in Three Fish Species in the Southern Gulf of St
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Barndoor Skate, Dipturus Laevis, Life History and Habitat Characteristics
NOAA Technical Memorandum NMFS-NE-173 Essential Fish Habitat Source Document: Barndoor Skate, Dipturus laevis, Life History and Habitat Characteristics U. S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service Northeast Region Northeast Fisheries Science Center Woods Hole, Massachusetts March 2003 Recent Issues in This Series: 155. Food of Northwest Atlantic Fishes and Two Common Species of Squid. By Ray E. Bowman, Charles E. Stillwell, William L. Michaels, and Marvin D. Grosslein. January 2000. xiv + 138 p., 1 fig., 7 tables, 2 app. NTIS Access. No. PB2000-106735. 156. Proceedings of the Summer Flounder Aging Workshop, 1-2 February 1999, Woods Hole, Massachusetts. By George R. Bolz, James Patrick Monaghan, Jr., Kathy L. Lang, Randall W. Gregory, and Jay M. Burnett. May 2000. v + 15 p., 5 figs., 5 tables. NTIS Access. No. PB2000-107403. 157. Contaminant Levels in Muscle of Four Species of Recreational Fish from the New York Bight Apex. By Ashok D. Deshpande, Andrew F.J. Draxler, Vincent S. Zdanowicz, Mary E. Schrock, Anthony J. Paulson, Thomas W. Finneran, Beth L. Sharack, Kathy Corbo, Linda Arlen, Elizabeth A. Leimburg, Bruce W. Dockum, Robert A. Pikanowski, Brian May, and Lisa B. Rosman. June 2000. xxii + 99 p., 6 figs., 80 tables, 3 app., glossary. NTIS Access. No. PB2001-107346. 158. A Framework for Monitoring and Assessing Socioeconomics and Governance of Large Marine Ecosystems. By Jon G. Sutinen, editor, with contributors (listed alphabetically) Patricia Clay, Christopher L. Dyer, Steven F. Edwards, John Gates, Tom A. Grigalunas, Timothy Hennessey, Lawrence Juda, Andrew W. Kitts, Philip N. -

Leucoraja Erinacea
Little Skate − Leucoraja erinacea Overall Vulnerability Rank = Low Biological Sensitivity = Low Climate Exposure = High Data Quality = 88% of scores ≥ 2 Expert Data Expert Scores Plots Leucoraja erinacea Scores Quality (Portion by Category) Low Moderate Stock Status 2.0 2.8 High Other Stressors 1.5 1.4 Very High Population Growth Rate 2.9 2.4 Spawning Cycle 1.2 3.0 Complexity in Reproduction 1.3 2.2 Early Life History Requirements 1.1 3.0 Sensitivity to Ocean Acidification 1.5 2.8 Prey Specialization 1.2 3.0 Habitat Specialization 1.2 3.0 Sensitivity attributes Sensitivity to Temperature 2.1 3.0 Adult Mobility 2.3 2.2 Dispersal & Early Life History 1.9 2.8 Sensitivity Score Low Sea Surface Temperature 3.9 3.0 Variability in Sea Surface Temperature 1.0 3.0 Salinity 2.0 3.0 Variability Salinity 1.2 3.0 Air Temperature 1.0 3.0 Variability Air Temperature 1.0 3.0 Precipitation 1.0 3.0 Variability in Precipitation 1.0 3.0 Ocean Acidification 4.0 2.0 Exposure variables Variability in Ocean Acidification 1.0 2.2 Currents 2.1 1.0 Sea Level Rise 1.1 1.5 Exposure Score High Overall Vulnerability Rank Low Little Skate (Leucoraja erinacea) Overall Climate Vulnerability Rank: Low (88% certainty from bootstrap analysis). Climate Exposure: High. Two exposure factors contributed to this score: Ocean Surface Temperature (3.9) and Ocean Acidification (4.0). Little Skate are demersal and complete their life cycle in marine habitats. Biological Sensitivity: Low. Only one attribute scored above 2.5: Population Growth Rate (3.4). -
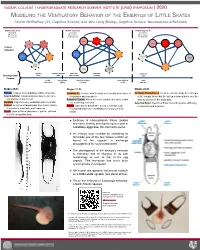
Modeling the Ventilatory Behavior of the Embryos Of
VASSAR COLLEGE | UNDERGRADUATE RESEARCH SUMMER INSTITUTE (URSI) SYMPOSIUM | 2020 MODELING THE VENTILATORY BEHAVIOR OF THE EMBRYOS OF LITTLE SKATES Connor McShaffrey (‘21, Cognitive Science) and John Long (Biology, Cognitive Science, Neuroscience & Behavior) retract freeze recoil retract freeze recoil retract recoil ventilate pulsate (L) select & pulsate ventilate return to enter tendril search ventilate & enter front- search return to faced & enter tendril Stages 30-31 Stages 31-32 Stages 32-33 Pulsate: Gradual, slow undulation without insertion. Ventilate (L): Ventilate with the body bent laterally at the base of Ventilate Front-Faced: Ventilate with the body bent sharply Search & Enter: Rapid undulation down to tail tip to the putative adult caudal fin. at the caudal fin so that the tail tip is looped back over the find and insert into a tendril. Return to Tendril: After a full recoil, return to the same tendril anterior portion of the body disk.. Ventilate: High frequency undulation within a tendril. that was being ventilated. Select & Enter: Search & Enter, but with greater efficiency Retract : An incremental pull-back from tendril relative Freeze: Upon partial disturbance during ventilation, stop of selection and insertion. to sensitivity, amplitude, and frequency. moving and wait before ventilating or moving out of the Recoil: Upon sufficient disturbance, pull the tail back tendril. to coil it around the body. ● Embryos of elasmobranch fishes (skates and some sharks) develop for up to a year in a leathery egg case, the mermaid’s purse. ● An embryo must ventilate by undulating its tail inside one of the four hollow tendrils (or horns) of the capsule to exchange deoxygenated for oxygenated water. -

Leucoraja Naevus from Portuguese Continental Waters
Universidade do Algarve Faculdade de Ciências e Tecnologia Reproductive biology of the species Leucoraja naevus from Portuguese continental waters Catarina Maia Master thesis submitted for the partial fulfillment of the title of Master of Marine Biology 2010 Universidade do Algarve Faculdade de Ciências e Tecnologia Reproductive biology of the species Leucoraja naevus from Portuguese continental waters Catarina Maia Master thesis submitted for the partial fulfillment of the title of Master of Marine Biology Internal supervisor: Prof. Dr. Karim Erzini External supervisor: Profa. Dra. Ivone Figueiredo 2010 Acknowledgements I would like to thank everyone who helped me in IPIMAR and University: First I would like to thank Dr. Ivone Figueiredo and Dr. Karim Erzini for the opportunity to perform this work and the availability and encouragement shown over the same; I would also like to express my immense gratitude to Dr. Barbara Serra-Pereira for the help, encouragement and support (tireless!!!!) that greatly facilitated my work; My sincere thanks to José do Lago and Neide Lagarto for their help in sampling and friendship; As Teresa, Ana Rita and Inês, Miguel and Nuno, who not only gave me the motivation but also by the availability and friendship shown. I also thank to all IPIMAR workers, including Carmo and Cristrina for their help and suggestions in histology; Tanks to PNAB that partially supported my work; My eternal gratitude to my parents and Francisco who were always by my side and supported me unconditionally. Abstract Skate populations tend to be highly vulnerable to exploitation as a result of the main life history characteristics (slow growth, late maturity and low fecundity). -

Batoid Locomotion: Effects of Speed on Pectoral Fin Deformation in the Little Skate, Leucoraja Erinacea Valentina Di Santo1,*, Erin L
© 2017. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2017) 220, 705-712 doi:10.1242/jeb.148767 RESEARCH ARTICLE Batoid locomotion: effects of speed on pectoral fin deformation in the little skate, Leucoraja erinacea Valentina Di Santo1,*, Erin L. Blevins1,2 and George V. Lauder1 ABSTRACT more efficient at higher speeds and for long-distance translocations Most batoids have a unique swimming mode in which thrust is (Di Santo and Kenaley, 2016). Although many batoid species are generated by either oscillating or undulating expanded pectoral fins accurately described by these two extreme modes, several species that form a disc. Only one previous study of the freshwater stingray has fall into a continuum between 0.5 and 1.0 wave, and are defined as quantified three-dimensional motions of the wing, and no comparable ‘semi-oscillators’ (Schaefer and Summers, 2005). data are available for marine batoid species that may differ The mechanics of propulsion in cartilaginous fishes have been considerably in their mode of locomotion. Here, we investigate three- investigated over the years through studies of morphology, dimensional kinematics of the pectoral wing of the little skate, kinematics, hydrodynamics, muscle activity and energetics Leucoraja erinacea, swimming steadily at two speeds [1 and (Daniel, 1988; Di Santo and Kenaley, 2016; Donley and 2 body lengths (BL) s−1]. We measured the motion of nine points in Shadwick, 2003; Fontanella et al., 2013; Lauder, 2015; Lauder three dimensions during wing oscillation and determined that there are and Di Santo, 2015; Porter et al., 2011; Rosenberger and Westneat, significant differences in movement amplitude among wing locations, 1999; Rosenblum et al., 2011). -

Metabolic Rate of Embryonic Little Skate, Raja Erinacea (Chondrichthyes: Batoidea): the Cost of Active Pumping 1 2 3,4 JILL B.K
JOURNAL OF EXPERIMENTAL ZOOLOGY 283:13–18 (1999) Metabolic Rate of Embryonic Little Skate, Raja erinacea (Chondrichthyes: Batoidea): The Cost of Active Pumping 1 2 3,4 JILL B.K. LEONARD, ADAM P. SUMMERS, * AND THOMAS J. KOOB 1S.O. Conte Anadromous Fish Research Center, Biological Resources Division, U.S. Geological Service, Turners Falls, Massachusetts 01376 2Organismic and Evolutionary Biology, University of Massachusetts, Amherst, Massachusetts 01003 3Skeletal Biology Section, Shriners Hospital for Children, Tampa, Florida 33612 4Mount Desert Island Biological Laboratory, Salsbury Cove, Maine 04672 ABSTRACT Near-hatching embryonic little skates, Raja erinacea, are highly active within their egg capsules, displaying a characteristic tail beating, which pumps water through the cap- sule. We measured the metabolic rate of late-stage embryos to determine whether oxygen suffi- cient for the embryo’s needs will diffuse through the egg capsule, and to assess the energetic cost of tail beating. Metabolic rate was inferred from oxygen consumption rates while embryos were in the capsules, unencapsulated, and anesthetized and unencapsulated. Anesthesia inhibited volun- tary movements, including tail wagging, allowing an estimate of the standard metabolic rate (SMR). –1 –1 Averaged over five embryos, the SMR was 0.032 ± 0.004 ml O2 g hr . There was no significant –1 –1 difference in metabolic rate between encapsulated (0.058 ± 0.009 ml O2 g hr ) and unencapsu- –1 –1 lated (0.049 ± 0.009 ml O2 g hr ) skates. Tail beating was found to be energetically expensive, requiring a 53%–81% increase over the SMR. From literature values for the oxygen permeability of the egg capsule we conclude that tail beating is required to supply sufficient oxygen to the embryonic skate. -

Sharks, Skates, Rays, and Chimaeras
SHARKS, SKATES, RAYS, AND CHIMAERAS UNITED STATES DEPARTMENT OF THE INTERIOR FISH AND WILDLIFE SERVICE BUREAU OF COMMERCIAL FISHERIES Circular 228 TABLE 1. -- tiximum sizes of camnon species of sharks Species Traditional Mucimum length Muimum length maximum size (measure<l--U. S. coa.ts) (recorde<l--world) Scientific na.rr;e from literature SixgL. st.ark .... 1 Hexanchus sp. .•..•••••••. 15 feet 5 inches 26 feet 5 inches nd hary... ..... Carcharias taurus... 10 feet 5 inches 12 feet 3 inches 15 feet 11 inches Porbeagle •....... 1 LamTUl TUlSUS........... ... 10 feet 12 feet 12 feet Sall10n shark. .... LamTUl ditropis . 8 feet 6 inches 8 feet 6 inches 12 feet L 0 .•.••.•.•.... Isurus oxyrinchus ...... ... 10 feet 6 inches 12 feet 12 feet - 13 feet 'hi te sr.ark. ..... Carcharodan carcharias. 18 feet 2 inches 21 feet 36 feet 6 inches Basking shar".... Cetorhinus maximus . 32 feet 2 inches 45 feet 40 feet - 50 feet Thresher shark... Alopias vulpinus . 18 feet 18 feet 20 feet rse shark...... Ginglymostoma cirraturn.. 9 feet 3 inches 14 feet Whale shark. ..... Rhincodan typus........ .•. 38 feet 45 feet 45 feet - 50 feet Olain dogfish.... Scyliorhinus retifer. ... .. 1 foot 5 inches 2 feet 6 inches Leopard shark.... Triakis semifasciata... 5 feet 5 feet Smooth dogfish ... Alustelus canis ......... ... 4 feet 9 inches 5 feet rieer shark...... Galeocerdo cuvieri..... ... 13 feet 10 inches 18 feet 30 feet Soupfin shark.... Galeorhinus zyopterus . .. 6 feet 5 inches 6 feet 5 inches 6 feet 5 inches Blue shark. ...... Prionace glauca ....... 11 feet 12 feet 7 inches 25 feet Bul .. shark. ...... Carcharhinus leucas. .. 9 feet 10 inches 10 feet Whi tetip shark. -

Finfish of Jamaica
Sampling Stations — Jamaica Bay Finfish Inventory Recreational Fishing Survey Gateway National Finfish of Recreation Area: 1985-1986 Jamaica Based on interviews of 450 fishermen, fishing the shores or bridges of Jamaica Bay: 1. The average number of years fished Jamaica Bay : 13 years. 2. When asked importance of "fishing for food" as a reason to fish on Jamaica Bay; 46 respondents said it was very important, 86 important, and 206 not impor tant. 112 persons did not respond. 3. When asked, "Do you eat fish caught in Jamaica Bay," 304 persons said Yes, 139 said No, and 7 did not respond. 4. People who eat fish from Jamaica Bay indicated that an average of 2.4 family members also eat Jamaica Bay fish. 5. The 304 persons who said they consume fish from Jamaica Bay were asked which species of fish they eat. The respondents answered as follows: bluefish, 89; winter flounder, 88; summer flounder, 77; porgy, 57; blackfish, 22; weakfish, 11; striped bass, 6; American eel, 5; black sea bass, 5; menhaden, 1; herring, 1. Total Number of Each Fish Species Captured by Otter Trawl, Gill Net, and Beach Seine in Jamaica Bay, November 1985 to October 1986 Compiled by: Smooth dogfish 37 White hake 2 Yellow jack 1 Butterfish 12 Little skate 2 Mummichog 210 Crevalle jack 2 Striped searobin 71 Acknowledgments Don Riepe Cownose ray 1 Striped killifish 700 Lookdown 2 Grubby 29 This list was compiled with the help of many National John T. Tanacredi, Ph.D. American eel 5 Atlantic Scup (porgy) 229 Smallmouth flounder 22 Park Service staff and volunteers. -

Long Island Sound Ecological Assessment —Appendices Alewife
The Long IslandSound Ecological Assessment APPENDICES table of contents Appendix A: Detailed Methodologies ...............................................6 i. Organism Classification .................................................................................. 6 ii. Sediment Interpolation .................................................................................. 7 Appendix B: Additional Data and Figures ................................................... 8 i. Physical Data ...................................................................................................... 8 Fall Average Dissolved Oxygen ................................................................ 9 Spring Average Dissolved Oxygen ........................................................... 10 Average Distance to River Outfalls ......................................................... 11 Fall Average Bottom Salinity ..................................................................... 12 Spring Average Bottom Salinity ............................................................... 13 Fall Average Surface Salinity ...................................................................... 14 Spring Average Surface Salinity ................................................................ 15 Fall Average Secchi Depth .......................................................................... 16 Spring Average Secchi Depth .................................................................... 17 Fall Average Bottom Temperature .......................................................... -

Striped Bass, Regarding the Following: A
RI Department of Environmental Management Marine Fisheries INFORMATIONAL PRE-HEARING WORKSHOP February 9, 2016 Marine Fisheries Informational Workshop – Rules of Engagement RIDFW staff will provide presentation on each of the proposed regulation amendments; Discussion will ensue upon completion of each presentation. Discussion should focus on: Understanding of the rationale behind each of the proposed amendments; Recommendations of alternatives. IMPORTANT! The workshop discussion is not an official public record. Please offer your preferred option(s) and comments for the public hearing record during the public hearing on February 16th. Refer to full annotated regulations provided on Division and Sec. of State websites; Public comment period will end at 4:00PM on Friday, February 26th. Public Hearing Items 1. Proposed amendments to RIMFR-Finfish regarding the following: a. Recreational Summer Flounder; b. Recreational Tautog; c. Commercial Tautog; d. Recreational Scup; e. Recreational Black Sea Bass; f. Commercial Coastal Sharks; g. Skate; h. General editing. 2. Proposed amendments to RIMFR-Striped Bass, regarding the following: a. Recreational Striped Bass; b. Commercial Striped Bass general category; c. Commercial Striped Bass floating fish trap; d. Commercial striped bass tags; e. Striped bass fin clipping provision (in conjunction with hearing item # 3b.) Public Hearing Items cont’d: 3. Proposed amendments RIMFR-Fish/Shellfish Dealer Regulations, regarding the following: a. General editing for improved clarity and readability of the regulations; b. Striped bass fin clipping provision. 4. Proposed REPEAL of RIMFR Part 1 – Legislative Findings (in conjunction with hearing item # 5); 5. Proposed ADOPTION of RIMFR Part 1 – Definitions (in conjunction with hearing item # 4); 6. Proposed REPEAL of RIMFR Part 17 – Maps. -

Revised 2020 and Projected 2021 Black Sea Bass and Scup
2020-2021 Scup and Black Sea Bass Specifications Environmental Assessment, Regulatory Impact Review, and Regulatory Flexibility Act Analysis March 2020 Prepared by the Mid-Atlantic Fishery Management Council in cooperation with the National Marine Fisheries Service Mid-Atlantic Fishery Management Council 800 North State Street, Suite 201 Dover, DE 19901 (302) 674-2331 tel. (302) 674-5399 fax National Marine Fisheries Service 55 Great Republic Drive Gloucester, MA 01930 (978) 281-9315 tel. (978) 281-9135 fax Initial submission to NMFS: January 14, 2020 Revisions submitted to NMFS: March 5, 2020 1 1. EXECUTIVE SUMMARY This document was prepared by the Mid-Atlantic Fishery Management Council (the Council or MAFMC) in consultation with the National Marine Fisheries Service (NMFS). This document was developed in accordance with all applicable laws and statutes as described in section 8. The purpose of this action is to implement commercial quotas and recreational harvest limits (RHLs) for the scup and black sea bass fisheries for 2020-2021. These measures are necessary to prevent overfishing and ensure that annual catch limits (ACLs) are not exceeded. This document describes all evaluated management alternatives (section 5) and their expected impacts on four aspects of the affected environment, which are defined as valued ecosystem components (VECs; sections 6 and 7). The expected impacts of the alternatives on the VECs are derived from consideration of both the current conditions of the VECs and expected changes in fishing effort under each alternative. Summary of 2020-2021 Scup Quota and RHL Alternatives and Impacts The 2020-2021 scup alternatives are summarized in Table 1 and described in more detail in section 5.1. -

Essential Fish Habitat Mapper Results for Surry Power Station
Essential Fish Habitat (EFH) Mapper Results for Surry Power Station, Units 1 and 2 U.S. Nuclear Regulatory Commission Subsequent License Renewal Environmental Review [NMFS] National Marine Fisheries Service. 2019. Essential Fish Habitat Mapper [online application]. Search Report for Latitude = 37º11'56" N, Longitude = 77º20'8" W. Available at <https://www.habitat.noaa.gov/application/efhmapper/index.html> (accessed May 20, 2019). Es EsFeHn tViiaelw F isToho lHabit Data tMa aQpupeerry Tool Zoom: Extent: Location Query: 10 Min sq.: Help: Region Loading... - Select Region - Essential Fish Habitat Habitat Areas of Particular Concern EFH Areas Protected from Fishing DDD: 37.305 lat, -75.978 long 5/20/2019 title EFH Data Notice: Essential Fish Habitat (EFH) is defined by textual descriptions contained in the fishery management plans developed by the regional Fishery Management Councils. In most cases mapping data can not fully represent the complexity of the habitats that make up EFH. This report should be used for general interest queries only and should not be interpreted as a definitive evaluation of EFH at this location. A location-specific evaluation of EFH for any official purposes must be performed by a regional expert. Please refer to the following links for the appropriate regional resources. Greater Atlantic Regional Office Atlantic Highly Migratory Specie s Management Division Query Results Degrees, Minutes, Seconds: Latitude = 37º11'56" N, Longitude = 77º20'8" W Decimal Degrees: Latitude = 37.20, Longitude = -76.66 The query location intersects with spatial data representing EFH and/or HAPCs for the following species/management units. *** W A R N I N G *** Please note under "Life Stage(s) Found at Location" the category "ALL" indicates that one or more life stage of a species (the one or ones listed) is mapped as EFH at the queried location.