Sharp Altitudinal Gradients in Magellanic Sub-Antarctic Streams: Patterns Along a Fluvial System in the Cape Horn Biosphere
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter14.Pdf
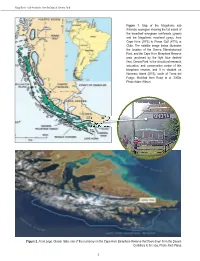
PART I • Omora Park Long-Term Ornithological Research Program THE OMORA PARK LONG-TERM ORNITHOLOGICAL RESEARCH PROGRAM: 1 STUDY SITES AND METHODS RICARDO ROZZI, JAIME E. JIMÉNEZ, FRANCISCA MASSARDO, JUAN CARLOS TORRES-MURA, AND RAJAN RIJAL In January 2000, we initiated a Long-term Ornithological Research Program at Omora Ethnobotanical Park in the world's southernmost forests: the sub-Antarctic forests of the Cape Horn Biosphere Reserve. In this chapter, we first present some key climatic, geographical, and ecological attributes of the Magellanic sub-Antarctic ecoregion compared to subpolar regions of the Northern Hemisphere. We then describe the study sites at Omora Park and other locations on Navarino Island and in the Cape Horn Biosphere Reserve. Finally, we describe the methods, including censuses, and present data for each of the bird species caught in mist nets during the first eleven years (January 2000 to December 2010) of the Omora Park Long-Term Ornithological Research Program. THE MAGELLANIC SUB-ANTARCTIC ECOREGION The contrast between the southwestern end of South America and the subpolar zone of the Northern Hemisphere allows us to more clearly distinguish and appreciate the peculiarities of an ecoregion that until recently remained invisible to the world of science and also for the political administration of Chile. So much so, that this austral region lacked a proper name, and it was generally subsumed under the generic name of Patagonia. For this reason, to distinguish it from Patagonia and from sub-Arctic regions, in the early 2000s we coined the name “Magellanic sub-Antarctic ecoregion” (Rozzi 2002). The Magellanic sub-Antarctic ecoregion extends along the southwestern margin of South America between the Gulf of Penas (47ºS) and Horn Island (56ºS) (Figure 1). -

Taiga Plains
ECOLOGICAL REGIONS OF THE NORTHWEST TERRITORIES Taiga Plains Ecosystem Classification Group Department of Environment and Natural Resources Government of the Northwest Territories Revised 2009 ECOLOGICAL REGIONS OF THE NORTHWEST TERRITORIES TAIGA PLAINS This report may be cited as: Ecosystem Classification Group. 2007 (rev. 2009). Ecological Regions of the Northwest Territories – Taiga Plains. Department of Environment and Natural Resources, Government of the Northwest Territories, Yellowknife, NT, Canada. viii + 173 pp. + folded insert map. ISBN 0-7708-0161-7 Web Site: http://www.enr.gov.nt.ca/index.html For more information contact: Department of Environment and Natural Resources P.O. Box 1320 Yellowknife, NT X1A 2L9 Phone: (867) 920-8064 Fax: (867) 873-0293 About the cover: The small photographs in the inset boxes are enlarged with captions on pages 22 (Taiga Plains High Subarctic (HS) Ecoregion), 52 (Taiga Plains Low Subarctic (LS) Ecoregion), 82 (Taiga Plains High Boreal (HB) Ecoregion), and 96 (Taiga Plains Mid-Boreal (MB) Ecoregion). Aerial photographs: Dave Downing (Timberline Natural Resource Group). Ground photographs and photograph of cloudberry: Bob Decker (Government of the Northwest Territories). Other plant photographs: Christian Bucher. Members of the Ecosystem Classification Group Dave Downing Ecologist, Timberline Natural Resource Group, Edmonton, Alberta. Bob Decker Forest Ecologist, Forest Management Division, Department of Environment and Natural Resources, Government of the Northwest Territories, Hay River, Northwest Territories. Bas Oosenbrug Habitat Conservation Biologist, Wildlife Division, Department of Environment and Natural Resources, Government of the Northwest Territories, Yellowknife, Northwest Territories. Charles Tarnocai Research Scientist, Agriculture and Agri-Food Canada, Ottawa, Ontario. Tom Chowns Environmental Consultant, Powassan, Ontario. Chris Hampel Geographic Information System Specialist/Resource Analyst, Timberline Natural Resource Group, Edmonton, Alberta. -

Modeling the Boundaries of Plant Ecotones of Mountain Ecosystems
Article Modeling the Boundaries of Plant Ecotones of Mountain Ecosystems Yulia Ivanova 1,* and Vlad Soukhovolsky 2 1 Institute of Biophysics, Siberian Branch, Russian Academy of Sciences, Federal Research Center “Krasnoyarsk Science Center SB RAS”, Academgorodok 50-50, 600036 Krasnoyarsk, Russia 2 V.N. Sukachev Institute of Forest, Siberian Branch, Russian Academy of Sciences, Federal Research Center “Krasnoyarsk Science Center SB RAS”, Academgorodok 50-28, 600036 Krasnoyarsk, Russia; [email protected] * Correspondence: [email protected]; Tel.: +7-391-249-4328; Fax: +7-391-243-3400 Academic Editors: Isabel Cañellas and Timothy A. Martin Received: 30 August 2016; Accepted: 5 November 2016; Published: 12 November 2016 Abstract: The ecological second-order phase transition model has been used to describe height-dependent changes in the species composition of mountain forest ecosystems. Forest inventory data on the distribution of various tree species in the Sayan Mountains (south Middle Siberia) are in good agreement with the model proposed in this study. The model was used to estimate critical heights for different altitudinal belts of vegetation, determine the boundaries and extents of ecotones between different vegetation belts, and reveal differences in the ecotone boundaries between the north- and south-facing transects. An additional model is proposed to describe ecotone boundary shifts caused by climate change. Keywords: ecotone; boundaries of ecotones; mountain forest ecosystems; biodiversity 1. Introduction Any plant community has its own species composition and spatial structure. Within the space occupied by the community, it may be regarded as uniform and characterized by spatially invariable parameters. The boundaries of a community are determined by the effects of external modifying factors (such as temperature) on the plants and competitive interactions between the species that are not characteristic of this community but are present in the neighboring one [1,2]. -

Argentine and Chilean Claims to British Antarctica. - Bases Established in the South Shetlands
Keesing's Record of World Events (formerly Keesing's Contemporary Archives), Volume VI-VII, February, 1948 Argentine, Chilean, British, Page 9133 © 1931-2006 Keesing's Worldwide, LLC - All Rights Reserved. Argentine and Chilean Claims to British Antarctica. - Bases established in the South Shetlands. - Chilean President inaugurates Chilean Army Bases on Greenwich Island. - Argentine Naval Demonstration in British Antarctic Waters. - H.M.S. "Nigeria" despatched to Falklands. - British Government Statements. - Argentine-Chilean Agreement on Joint Defence of "Antarctic Rights." - The Byrd and Ronne Antarctic Expeditions. - Australian Antarctic Expedition occupies Heard Islands. The Foreign-Office in London, in statements on Feb. 7 and Feb. 13, announced that Argentina and Chile had rejected British protests, earlier presented in Buenos Aires and Santiago, against the action of those countries in establishing bases in British Antarctic territories. The announcement of Feb. 7 stated that on Dec. 7, 1947, the British Ambassador in Buenos Aires, Sir Reginald Leeper, had presented a Note expressing British "anxiety" at the activities in the Antarctic of an Argentine naval expedition which had visited part of the Falkland Islands Dependencies, including Graham Land, the South Shetlands, and the South Orkneys, and had landed at various points in British territory; that a request had been made for Argentine nationals to evacuate bases established on Deception Island and Gamma Island, in the South Shetlands; that H.M. Government had proposed that the Argentine should submit her claim to Antarctic sovereignty to the International Court of Justice for adjudication; and that on Dec. 23, 1947, a second British Note had been presented expressing surprise at continued violations of British territory and territorial waters by Argentine vessels in the Antarctic. -

Invaders Without Frontiers: Cross-Border Invasions of Exotic Mammals
Biological Invasions 4: 157–173, 2002. © 2002 Kluwer Academic Publishers. Printed in the Netherlands. Review Invaders without frontiers: cross-border invasions of exotic mammals Fabian M. Jaksic1,∗, J. Agust´ın Iriarte2, Jaime E. Jimenez´ 3 & David R. Mart´ınez4 1Center for Advanced Studies in Ecology & Biodiversity, Pontificia Universidad Catolica´ de Chile, Casilla 114-D, Santiago, Chile; 2Servicio Agr´ıcola y Ganadero, Av. Bulnes 140, Santiago, Chile; 3Laboratorio de Ecolog´ıa, Universidad de Los Lagos, Casilla 933, Osorno, Chile; 4Centro de Estudios Forestales y Ambientales, Universidad de Los Lagos, Casilla 933, Osorno, Chile; ∗Author for correspondence (e-mail: [email protected]; fax: +56-2-6862615) Received 31 August 2001; accepted in revised form 25 March 2002 Key words: American beaver, American mink, Argentina, Chile, European hare, European rabbit, exotic mammals, grey fox, muskrat, Patagonia, red deer, South America, wild boar Abstract We address cross-border mammal invasions between Chilean and Argentine Patagonia, providing a detailed history of the introductions, subsequent spread (and spread rate when documented), and current limits of mammal invasions. The eight species involved are the following: European hare (Lepus europaeus), European rabbit (Oryctolagus cuniculus), wild boar (Sus scrofa), and red deer (Cervus elaphus) were all introduced from Europe (Austria, France, Germany, and Spain) to either or both Chilean and Argentine Patagonia. American beaver (Castor canadensis) and muskrat (Ondatra zibethicus) were introduced from Canada to Argentine Tierra del Fuego Island (shared with Chile). The American mink (Mustela vison) apparently was brought from the United States of America to both Chilean and Argentine Patagonia, independently. The native grey fox (Pseudalopex griseus) was introduced from Chilean to Argentine Tierra del Fuego. -

2.2 Ecosystems of the World E
Ecotoxicology and Climate Edited by P. Bourdeau, J. A. Haines, W. Klein and C. R. Krishna Murti @ 1989 SCOPE. Published by John Wiley & Sons Ltd 2.2 Ecosystems of the World E. F. BRUENIG 2.2.1 DEFINITION An ecosystem is a community of organisms and their physical and chemical environment interacting as an ecological unit. It represents all biological and abiotic components, including man, within a defined and delimited biotope, and is characterized by distinct ecological biocoenotic features of structure and functioning. The ecosystem relates to many different scales. At the lowest end of the scale, the ecosystem may comprise a rock, a fallen tree, or a certain layer in a plant community (pico-ecosystem, as described by Ellenberg, 1973). At the largest end of the scale it relates to life support media and covers oceanic, terrestrial, limnic, or urban ecosystem categories (mega-ecosystem). The present description is restricted to terrestrial ecosystems and focuses in particular on potential natural vegetation. Centred on the plant formation, it classes natural forests and woodlands in hot and cold climates at macro-ecosystem level. The description is structured by classifying the forests and woodlands at micro- ecosystem (formation group) level according to major physiognomic, pheno- logical, and growth features in relation to the site and environmental conditions. These units correspond roughly to the bioclimatic life zones of Holdridge (1967). The description is patterned on the serial structure of the physiognomic- ecological classification of plant formations of the earth (Miiller-Dombois and Ellenberg, 1974, pp. 466-488) commonly quoted as the UNESCO Classification. 2.2.2 NATURAL VEGETATIONAL ZONES 2.2.2.1 Latitudinal Zonation The characteristic features of the zonal vegetation in each latitudinal zone are primarily determined and shaped by climate. -

First Evidence of Insect Attraction by a Southern Hemisphere Splachnaceae: the Case of Tayloria Dubyi Broth
Nova Hedwigia Vol. 92 issue 3–4, 317–326 Article Stuttgart, May 2011 First evidence of insect attraction by a Southern Hemisphere Splachnaceae: The case of Tayloria dubyi Broth. in the Reserve Biosphere Cape Horn, Chile. Jocelyn Jofre1*, Bernard Goffinet3, Paul Marino4, Robert A. Raguso5, Silvio Shigueo Nihei6, Francisca Massardo1,2 and Ricardo Rozzi1,2,7 1 Instituto de Ecología y Biodiversidad, Facultad de Ciencias, Universidad de Chile, Casilla 653, Santiago, Chile. [email protected] 2 Programa de Magíster en Ciencias, Facultad de Ciencias, Universidad de Magallanes, Casilla 113-D, Avenida Bulnes 01855, Punta Arenas, Chile 3 Department of Ecology and Evolutionary Biology, 75 N. Eagleville Road, University of Connecticut, Storrs, CT 06269-3043, USA 4 Department of Biology, Memorial University, St. John´s, NL A1B 3X9, Canada 5 Department of Neurobiology and Behavior, Seeley G. Mudd Hall, Cornell University, Ithaca, NY 14853-2702, USA 6 Departamento de Zoologia, Instituto de Biociências – Universidade de São Paulo, Rua do Matão, Trav. 14, n. 101, 05508-900, São Paulo/SP, Brasil. 7 Department of Philosophy, University of North Texas, Denton, TX 76201 With 3 figures and 1 table Jofre, J., B. Goffinet, P. Marino, R.A. Raguso, S.S. Nihei, F. Massardo & R. Rozzi (2011): First evidence of insect attraction by a Southern Hemisphere Splachnaceae: The case of Tayloria dubyi Broth. in the Reserve Biosphere Cape Horn, Chile. – Nova Hedwigia 92: 317–326. Abstract: The moss Tayloria dubyi (Splachnaceae) is endemic to the subantarctic Magallanes ecoregion where it grows exclusively on bird dung and perhaps only on feces of the goose Chloephaga picta, a unique habitat among Splachnaceae. -

With National Geographic
Around Cape Horn & the Chilean Fjords - with National Geographic From 02/03/2021 From Ushuaia Ship: LE BOREAL to 14/03/2021 to Talcahuano In partnership with National Geographic Expeditions. Discover Patagonia during a 13-day expedition cruise. PONANT is offering you the opportunity to explore the most beautiful scenery Argentina and Chile have to offer, including glaciers, fjords, and winding channels. You will embark in Ushuaia, the world’s southernmost city, and round the mythical Cape Horn. Puerto Williams, considerated as the southernmost village in the world, will be your first port of call. Next enter the magic of the Chilean fjords and channels for exceptional moments spent sailing amidst landscapes of unrivalled beauty. The narrow passages, channels and fjords will lead you right up close to stunning glaciers, including Garibaldi, Agostini, El Brujo, Overnight in Buenos Aires + flight Buenos Pie XI. Aires/Ushuaia + visit + flight Concepcion/Santiago Your ship will reach Tortel and its charming stilt houses interconnected by a labyrinth of wooden footbridges. During the last part of your cruise in South America, you will call at Quemchi, after a sailing during which it is not unusual to encounter sea lions and porpoises. Quemchi is an authentic village located on the lush island of Chiloé. Subject to ice and weather conditions. The expedition highlights and itineraries described above illustrate possible experiences only and cannot be guaranteed. The information in this document is valid as of 11/08/2020 Around Cape Horn & the Chilean Fjords - with National Geographic YOUR STOPOVERS : USHUAIA Embarkation 02/03/2021 from 16h00 to 17h00 Departure 02/03/2021 at 18h00 Capital of Argentina's Tierra del Fuego province, Ushuaia is considered the gateway to the White Continent and the South Pole. -

Sharp Altitudinal Gradients in Magellanic Sub-Antarctic Streams: Patterns Along a Fluvial System in the Cape Horn Biosphere Rese
Author's personal copy Polar Biol DOI 10.1007/s00300-015-1746-4 ORIGINAL PAPER Sharp altitudinal gradients in Magellanic Sub-Antarctic streams: patterns along a fluvial system in the Cape Horn Biosphere Reserve (55°S) 1,2,3,6 1,2,3,4 1,2,3,5,6 Tamara Contador • James H. Kennedy • Ricardo Rozzi • Jaime Ojeda Villarroel1,2,3,6 Received: 17 July 2014 / Revised: 20 June 2015 / Accepted: 22 June 2015 Ó Springer-Verlag Berlin Heidelberg 2015 Abstract Magellanic Sub-Antarctic streams run through Antarctic watershed is characterized by a sharp thermal steep, low-altitude mountainous gradients characterized by gradient, in which cumulative degree-days (°C) per year a topography that supports a mosaic of evergreen, mixed, sharply increase through a relatively short altitudinal gra- and deciduous forests, peat lands, and scrublands. Here, the dient (0–600 m above sea level). With the results from this macroinvertebrate fauna and their ecological interactions study, we can now study thermal tolerances of altitude- are poorly known. This study linked the distribution, restricted species or project changes in their distributions community composition, and functional feeding structure and voltinism patterns according to climate change sce- of benthic macroinvertebrates with physicochemical and narios. Ecosystems at higher latitudes and altitudes are thermal patterns along the altitudinal gradient of a Mag- experiencing some of the fastest rates of warming on the ellanic Sub-Antarctic watershed. Invertebrates were col- planet, and Magellanic Sub-Antarctic watersheds could be lected during the austral summers of 2008, 2009, and 2010 considered as ‘‘sentinel systems,’’ providing early warning at five different altitudes. -

Dientes De Navarino Circuit TREK 7 Days Wilderness Trekking on the Isla Navarino - Tierra Del Fuego
Dientes de Navarino Circuit TREK 7 days Wilderness Trekking on the Isla Navarino - Tierra del Fuego The “Dientes de Navarino Circuit” is a trekking experience at the edge of the world! This southernmost trekking route is a once-in-a-lifetime experience that will fascinate curious and experienced hikers. On this pioneer-adventure in the mountains of Dientes de Navarino, you will land right in the middle of Tierra del Fuego’s mystic beauty. Hike through wet swampland and quaint forests of beech trees before you finally reach the sharp teeth, Los Dientes, of Navarino Island. These natural jewels are embedded in small blue-lustrous lagoons. Also hike through the southern end of the world to watch beavers building their dams, while resisting the extremely strong gusts of wind that indicate the closeness of Cape Horn. Trip Highlights: • Hike the southernmost trek at the edge of the world, the Dientes de Navarino Circuit on Isla Navarino • Set up camp between rock pinnacles, alpine lakes & beaver dams • Visit the charming fishing village Puerto Williams, the most southerly village in the world • Fly over the legendary Strait of Magellan, Darwin mountain range and Beagle-Channel • Enjoy a freshly caught king crab prepared by a local family Insider Tip: • Take a trip from Ushuaia to Martillo Island and learn more about see lions, dolphins and Magellan penguins Trip Info: Trip length: 7 days Start/End of the trip: from / to Punta Arenas or Ushuaia Group Size: min. 3 / max. 12 people Departures: see set departures on our website or individual on -

Mangrove Assessment in Manamoc Island for Coastal Retreat Mitigation
Journal of Marine and Island Cultures, v7n1 — Martinez & Buot Mangrove assessment in Manamoc Island for coastal retreat mitigation Mylene R. Martinez Inocencio E. Buot Jr. (corresponding author) School of Environmental Science and Management, University of the Philippines Los Baños, College, Laguna School of Environmental Science and Management, University of Institute of Biological Sciences, College of Arts and Sciences, the Philippines Los Baños, College, Laguna University of the Philippines Los Baños, College, Laguna [email protected] Faculty of Management and Development Studies, University of the Philippines Open University, Los Baños, Laguna Publication Information: Received 5 April 2018, Accepted 11 May 2018, Available online 30 June 2018 DOI: 10.21463/jmic.2018.07.1.05 Abstract Manamoc Island is experiencing coastline retreat and is in urgent need of economical mitigating measures. This study explored the role of mangroves in the mitigation of coastal retreat in Manamoc Island. Assessment of mangroves through standard vegetation analysis was done in relation to the topography and coastal dynamics prevalent in Manamoc Island. Time series analysis of sand and mangrove cover change was carried out to determine the role of mangroves in coastal retreat mitigation. Cluster analysis revealed five clusters named after the dominant species: Cluster I – Avicennia marina (Forsk.) Vierh.; Cluster II – Bruguiera sexangula (Lour.) Poir.; Cluster III – Rhizophora apiculata Blume and Rhizophora mucronata Lam.; Cluster IV – Rhizophora mucronata Lam.; and Cluster V – Sonneratia alba J. Smith. The clustering pattern followed that of typical mangrove zonation landward, middleward, and seaward although with a relatively short width. Canonical correspondence analysis (CCA) indicated that environmental factors like soil texture, pH, N, and P influenced mangrove distribution in Manamoc Island. -

Curriculum Vitae Abreviado
ABRIDGED CURRICULUM VITAE - March 2016 1. PERSONAL DATA Name, Christian names: RABASSA, Jorge Oscar Born in: La Plata, Argentina, on December 2nd. 1948 Argentine Passport N°: 05.400.236 M Home Street: Buenos Aires 2869, Barrio Los Alakalufes 2. City: V9410BFD. USHUAIA Province: Tierra del Fuego, Argentina Mobile telephone: +54-9-2901-6-19503 Office Phone + Fax: +54-2901-430644/422310/422314 2. EDUCATION Licenciado en Geología, 20/08/71. Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, La Plata, Argentina. Doctor en Ciencias Naturales (Geology), 23/12/74. Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata. 3. POSITIONS HELD IN EDUCATION AND RESEARCH Research and Science Management Director of CADIC, CONICET, from October 27th., 2011, onwards. Investigador Superior, CADIC, CONICET, from 1-10-10 up to date. President, Argentine Association of Geomorphology and Quaternary Studies (2015- 2018; affiliated to INQUA and IAG) Investigador Principal, CADIC, CONICET, 1-1-95 al 1-10-10. Minister of Education, Culture, Science and Technology, province of Tierra del Fuego, December 2007 - june 2008. Member of the Earth Sciences Committee, CONICET, april 2006 - november 2007, and 2004. Rector, Universidad Nacional del Comahue. 1998-2002 Investigador Independiente, CADIC, CONICET, 1988-1994. Director, CADIC, Centro Austral de Investigaciones Científicas, CONICET, Ushuaia, 1986-1990. Investigador Independiente, Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, 1985-1988. Investigador Principal, Instituto Antártico Argentino, Programa HIELOANTAR, Proyecto GEOGLA-Ross, Summer seasons 1980-1981-1982. Investigador Adjunto, Comisión de Investigaciones Científicas de la Provincia de Buenos Aires, 1979-1985. Fulbright Post-doctoral Fellow and Senior Research Associate, Department of Geological Sciences, State University of New York at Binghamton, Binghamton, New York, U.S.A., 1975-1976.