Rothschild & Co Risk-Based US Index
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prostate Cancer in Focus
PROSTATE CANCER IN FOCUS Current Developments in the Management of Prostate Cancer Section Editor: Andrew J. Armstrong, MD Prostate Cancer Prostate PSMA-Targeted Therapy in Prostate Cancer Scott T. Tagawa, MD, MS Professor of Medicine and Urology Weill Cornell Medicine New York, New York H&O What is prostate-specific membrane agent such as a monoclonal antibody might be able to antigen (PSMA), and what makes it a good target target a tumor without binding to other PSMA-positive for the treatment of prostate cancer? sites owing to its large size and physical inability to reach luminal sites of expression that are separated from the ST PSMA is a cell surface antigen that is expressed to vasculature by tight junctions. a limited degree on certain normal cells in the body but generally is highly overexpressed in the setting of prostate H&O How is PSMA positivity established? cancer. Normal cells that express PSMA are located in the prostate, salivary, and lacrimal glands; the proximal part ST Imaging and biopsy are the main ways to establish of the small intestine; the proximal renal tubules; and PSMA positivity. Multiple imaging modalities are avail- some ganglia. In the setting of prostate cancer, PSMA able worldwide. The agent indium In 111 capromab expression generally increases along with the cancer grade, pendetide (ProstaScint) has been approved for years in and it also increases following hormonal therapy. In addi- the United States, and the US Food and Drug Adminis- tion, PSMA expression tends to be higher in metastatic tration (FDA) approved the use of gallium Ga 68 PSMA- sites than in primary tumors. -

Uniqure N.V. Paasheuvelweg 25A 1105BP Amsterdam the Netherlands +1-339-970-7000
uniQure N.V. Paasheuvelweg 25a 1105BP Amsterdam The Netherlands +1-339-970-7000 NOTICE OF EXTRAORDINARY GENERAL MEETING OF SHAREHOLDERS To be held on September 14, 2017 To the Shareholders of uniQure N.V.: Notice is hereby given that an Extraordinary General Meeting of Shareholders (the “Extraordinary Meeting”) of uniQure N.V., a public company with limited liability ( naamloze vennootschap ) under the laws of the Netherlands (the “Company,” “uniQure,” and “we”), will be held on September 14, 2017, at 9:30 a.m., Central European Summer Time, at the Company’s principal executive offices located at Paasheuvelweg 25a, 1105BP Amsterdam, the Netherlands, for the following purposes: I. Opening and announcements; II. Appointment of Jeremy P. Springhorn, Ph.D. as a non-executive director (voting proposal no. 1); III. Appointment of Madhavan Balachandran as a non-executive director (voting proposal no. 2); IV Any other business that may properly come before the meeting or any adjournment of the meeting; and V. Closing of the meeting. Each person authorized to attend the Extraordinary Meeting may inspect the Agenda at the office of uniQure. Our Board of Directors (our “Board”) recommends that you vote “FOR” each of the voting proposals noted above. The record date is set at the close of business on August 17, 2017 EST and, therefore, only the Company’s shareholders of record at the close of business on August 17, 2017 EST are entitled to receive this notice (this “Notice”) and to vote at the Extraordinary Meeting and any adjournment thereof. Only shareholders who have given notice in writing to the Company by September 12, 2017 of their intention to attend the Extraordinary Meeting in person are entitled to attend the Extraordinary Meeting in person. -

Keurig to Acquire Dr Pepper Snapple for $18.7Bn in Cash
Find our latest analyses and trade ideas on bsic.it Coffee and Soda: Keurig to acquire Dr Pepper Snapple for $18.7bn in cash Dr Pepper Snapple Group (NYSE:DPS) – market cap as of 17/02/2018: $28.78bn Introduction On January 29, 2018, Keurig Green Mountain, the coffee group owned by JAB Holding, announced the acquisition of soda maker Dr Pepper Snapple Group. Under the terms of the reverse takeover, Keurig will pay $103.75 per share in a special cash dividend to Dr Pepper shareholders, who will also retain 13 percent of the combined company. The deal will pay $18.7bn in cash to shareholders in total and create a massive beverage distribution network in the U.S. About Dr Pepper Snapple Group Incorporated in 2007 and headquartered in Plano (Texas), Dr Pepper Snapple Group, Inc. manufactures and distributes non-alcoholic beverages in the United States, Mexico and the Caribbean, and Canada. The company operates through three segments: Beverage Concentrates, Packaged Beverages, and Latin America Beverages. It offers flavored carbonated soft drinks (CSDs) and non-carbonated beverages (NCBs), including ready-to-drink teas, juices, juice drinks, mineral and coconut water, and mixers, as well as manufactures and sells Mott's apple sauces. The company sells its flavored CSD products primarily under the Dr Pepper, Canada Dry, Peñafiel, Squirt, 7UP, Crush, A&W, Sunkist soda, Schweppes, RC Cola, Big Red, Vernors, Venom, IBC, Diet Rite, and Sun Drop; and NCB products primarily under the Snapple, Hawaiian Punch, Mott's, FIJI, Clamato, Bai, Yoo- Hoo, Deja Blue, ReaLemon, AriZona tea, Vita Coco, BODYARMOR, Mr & Mrs T mixers, Nantucket Nectars, Garden Cocktail, Mistic, and Rose's brand names. -

Fund Holdings As of 6/30/2021 Massmutual Balanced Fund Invesco Prior to 5/1/2021, the Fund Name Was Massmutual Premier Balanced Fund
Fund Holdings As of 6/30/2021 MassMutual Balanced Fund Invesco Prior to 5/1/2021, the Fund name was MassMutual Premier Balanced Fund. Fund Shares or Par Position Market Security Name Ticker CUSIP Weighting % Amount Value Apple Inc AAPL 037833100 3.91 48,433 6,633,384 Microsoft Corp MSFT 594918104 3.45 21,552 5,838,437 USTREAS T-Bill Auction Ave 3 Mon 1.69 2,862,977 JPMorgan Chase & Co JPM 46625H100 1.56 16,948 2,636,092 Verizon Communications Inc VZ 92343V104 1.45 43,768 2,452,321 The Home Depot Inc HD 437076102 1.42 7,556 2,409,533 Intel Corp INTC 458140100 1.29 38,961 2,187,271 Procter & Gamble Co PG 742718109 1.04 13,105 1,768,258 Cisco Systems Inc CSCO 17275R102 1.03 32,830 1,739,990 UnitedHealth Group Inc UNH 91324P102 1.00 4,215 1,687,855 Comcast Corp Class A CMCSA 20030N101 0.94 28,021 1,597,757 AT&T Inc T 00206R102 0.91 53,587 1,542,234 Oracle Corp ORCL 68389X105 0.83 18,031 1,403,533 Deere & Co DE 244199105 0.76 3,635 1,282,101 Accenture PLC Class A ACN G1151C101 0.74 4,237 1,249,025 Johnson Controls International PLC JCI G51502105 0.74 18,185 1,248,037 Visa Inc Class A V 92826C839 0.71 5,152 1,204,641 Texas Instruments Inc TXN 882508104 0.70 6,128 1,178,414 Costco Wholesale Corp COST 22160K105 0.67 2,850 1,127,660 Bank of America Corp BAC 060505104 0.64 26,192 1,079,896 Broadcom Inc AVGO 11135F101 0.63 2,223 1,060,015 Abbott Laboratories ABT 002824100 0.57 8,348 967,784 Target Corp TGT 87612E106 0.56 3,949 954,631 Honeywell International Inc HON 438516106 0.56 4,324 948,469 Goldman Sachs Group Inc GS 38141G104 0.53 2,374 901,004 -

OLSTEIN ALL CAP VALUE FUND SCHEDULE of INVESTMENTS March 31, 2021 (Unaudited) COMMON STOCKS - 84.7% Shares Value Advertising Agencies - 1.4% Omnicom Group, Inc
OLSTEIN ALL CAP VALUE FUND SCHEDULE OF INVESTMENTS March 31, 2021 (Unaudited) COMMON STOCKS - 84.7% Shares Value Advertising Agencies - 1.4% Omnicom Group, Inc. 140,000 $ 10,381,000 Aerospace & Defense - 1.9% L3Harris Technologies, Inc. 39,000 7,904,520 Raytheon Technologies Corporation 77,000 5,949,790 13,854,310 Air Delivery & Freight Services - 2.4% FedEx Corporation 35,000 9,941,400 United Parcel Service, Inc. - Class B 45,000 7,649,550 17,590,950 Airlines - 2.1% Delta Air Lines, Inc. (a) 158,000 7,628,240 JetBlue Airways Corporation (a) 353,000 7,180,020 14,808,260 Auto Manufacturers - 1.0% General Motors Company (a) 129,000 7,412,340 Beverages - 0.6% Keurig Dr Pepper, Inc. (b) 124,000 4,261,880 Building Products - 1.2% Carrier Global Corporation 198,000 8,359,560 Capital Markets - 1.4% Goldman Sachs Group, Inc. 31,500 10,300,500 Chemicals - 1.8% Corteva, Inc. 201,000 9,370,620 Eastman Chemical Company 31,000 3,413,720 12,784,340 Commercial Banks - 5.8% Citizens Financial Group, Inc. 154,000 6,799,100 Fifth Third Bancorp 228,000 8,538,600 Prosperity Bancshares, Inc. 68,000 5,092,520 U.S. Bancorp 181,000 10,011,110 Wells Fargo & Company 280,000 10,939,600 41,380,930 Commercial Services - 1.6% Moody's Corporation 18,500 5,524,285 S&P Global, Inc. 17,000 5,998,790 11,523,075 Communications Equipment - 1.9% Cisco Systems, Inc. 266,000 13,754,860 Computers - 1.9% Apple, Inc. -

Interim Recommendations for Use of the Moderna Mrna-1273 Vaccine Against COVID-19
Interim recommendations for use of the Moderna mRNA-1273 vaccine against COVID-19 Interim guidance First issued 25 January 2021 Updated 15 June 2021 Background This interim guidance has been developed on the basis of the advice issued by the Strategic Advisory Group of Experts on Immunization (SAGE) at its extraordinary meeting on 21 January 2021 (1) and updated during its extraordinary meeting on 27 May 2021(2). Declarations of interests were collected from all external contributors and assessed for any conflicts of interest. Summaries of the reported interests can be found on the SAGE meeting website and SAGE Working Group website. The guidance is based on the evidence summarised in the Background document on the Moderna mRNA-1273 vaccine against COVID-19 (3) and the background paper on COVID-19 disease and vaccines (4). Annexes which include GRADE and evidence-to-recommendations (ETR) tables have also been updated to reflect the updated recommendations. All referenced documents are available on the SAGE COVID-19 webpage: https://www.who.int/groups/strategic- advisory-group-of-experts-on-immunization/covid-19-materials. These interim recommendations refer to the mRNA-1273 vaccine, manufactured by Moderna. The vaccine is also known as COVID-19 Vaccine Moderna. In the subsequent text the vaccine will be referred to as mRNA-1273. On 30 April 2021, mRNA-1273 was granted WHO’s Emergency Use Listing (EUL). Methods SAGE applies the principles of evidence-based medicine and has set in place a thorough methodological process for issuing and updating recommendations (5). A detailed description of the methodological processes as they apply to COVID-19 vaccines can be found in the SAGE evidence framework for COVID-19 vaccines (6). -

Why Keurig Is Acquiring Dr. Pepper (Rabobank)
January 2018 Why Keurig Is Acquiring Dr Pepper Breaking Down the Walls Between Coffee and Soft Drinks RaboResearch Food & Agribusiness far.rabobank.com Summary The strategic basis for the Keurig – DPS combination is clear: to create a company that Jim Watson combines hot/cold beverages and retail/office/e-commerce distribution in order to match the Senior Analyst - Beverages +1 212 916 7943 way its consumers experience the beverage world. The strategic side of the combination will take significant execution skill, while the financial side (creating synergies and an exit strategy) looks to be more straightforward. Details of the acquisition On January 29, 2018 Keurig agreed to a merger with Dr Pepper Snapple to form Keurig Dr Pepper (KDP). Dr Pepper Snapple shareholders will receive USD 103.75 per share (for a total cash value of USD 19 billion) and retain a 13% stake in the new public company. The total value to DPS shareholders represents an EV/EBITDA multiple of 17.6x. JAB, together with its partners, will make an equity investment of USD 9 billion to help finance this transaction. Coffee + soft drink opportunities and challenges KDP integrates hot and cold beverages to a degree we haven’t seen before, but we see this as a step towards a more combined coffee and soft-drink world. The most concrete example presented by the company of top-line synergies is the ability to increase distribution of ready-to- drink coffee products, with access to the broader JAB coffee portfolio. We wrote about the blurring lines between coffee and soft drinks in our note Coffee Joins the Beverage Party: What Happens when a Can of Coffee Looks Just Like a Can of Soda? We are bullish on the potential for ready-to-drink coffee in North America – and believe the new KDP could build a real challenger to Starbucks/Pepsi in this space. -

Carbonated Soft Drinks in the US Through 2025
Carbonated Soft Drinks in the U.S. through 2025: Market Essentials 2021 Edition (To be published September 2021. Data through 2020. Market projections through 2025.) More than 120 Excel tables plus an executive summary detailing trends and comprehensive profiles of leading companies, their brands and their strategies. his comprehensive market research report on the number-two T beverage category examines trends and top companies' For A Full strategies. It provides up-to-date statistics on leading brands, Catalog of packaging, quarterly growth and channels of distribution. It also Reports and offers data on regional markets, pricing, demographics, Databases, advertising, five-year growth projections and more. Go To The report presents the data in Excel spreadsheets, which it supplements with a concise executive summary highlighting key bmcreports.com developments including the impact of the coronavirus pandemic as well as a detailed discussion of the leading carbonated soft drink (CSD) companies. INSIDE: REPORT OVERVIEW A brief discussion of key AVAILABLE FORMAT & features of this report. 2 PRICING TABLE OF CONTENTS Direct Download A detailed outline of this Excel sheets, PDF, PowerPoint & Word report’s contents and data tables. 7 $4,395 To learn more, to place an advance order or to inquire about SAMPLE TEXT AND additional user licenses call: Charlene Harvey +1 212.688.7640 INFOGRAPHICS ext. 1962 [email protected] A few examples of this report’s text, data content layout and style. 13 HAVE Contact Charlene Harvey: 212-688-7640 x 1962 QUESTIONS? [email protected] Beverage Marketing Corporation 850 Third Avenue, 13th Floor, New York, NY 10022 Tel: 212-688-7640 Fax: 212-826-1255 The answers you need Carbonated Soft Drinks in the U.S. -
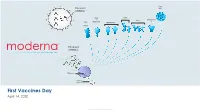
Moderna Vaccine Day Presentation
Zika Encoded VLP mRNA(s) RSV CMV + EBV SARS-CoV- RSV-ped 2 Flu hMPV/PIV3 H7N9 Encoded mRNA(s) Ribosome Protein chain(s) First Vaccines Day April 14, 2020 © 2020 M oderna Therapeutics Forward-looking statements and disclaimers This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended including, but not limited to, statements concerning; the impact of the SARS-CoV-2 pandemic on the Company’s clinical trials and operations; the timing and finalization of a dose-confirmation Phase 2 study and planning for a pivotal Phase 3 study for mRNA-1647; the status and outcome of the Phase 1 clinical trial for mRNA-1273 being conducted by NIH; the next steps and ultimate commercial plan for mRNA-1273; the size of the potential market opportunity for mRNA-1273; the size of the potential commercial market for novel vaccines produced by Moderna or others; the potential peak sales for the Company’s wholly-owned vaccines; the probability of success of the Company’s vaccines individually and as a portfolio; and the ability of the Company to accelerate the research and development timeline for any individual product or the platform as a whole. In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. The forward-looking statements in this press release are neither promises nor guarantees, and you should not place undue reliance on these forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, many of which are beyond Moderna’s control and which could cause actual results to differ materially from those expressed or implied by these forward-looking statements. -

Refreshing the Biologic Pipeline 2020
news feature Credit: Science Lab / Alamy Stock Photo Refreshing the biologic pipeline 2020 In the absence of face-to-face meetings, FDA and industry implemented regulatory workarounds to maintain drug and biologics approvals. These could be here to stay. John Hodgson OVID-19 might have been expected since 1996) — a small miracle in itself “COVID-19 confronted us with the need to severely impair drug approvals (Fig. 1 and Table 1). to better triage sponsors’ questions,” says Cin 2020. In the event, however, To the usual crop of rare disease and Peter Marks, the director of the Center for industry and regulators delivered a small genetic-niche cancer treatments, 2020 Biologics Evaluation and Research (CBER) miracle. They found workarounds and also added a chimeric antigen receptor at the FDA. “That was perhaps the single surrogate methods of engagement. Starting (CAR)-T cell therapy with a cleaner biggest takeaway from the pandemic related in January 2020, when the outbreak veered manufacturing process and the first to product applications.” Marks says that it westward, the number of face-to face approved blockbuster indication for a became very apparent with some COVID- meetings declined rapidly; by March, small-interfering RNA (siRNA) — the 19-related files that resolving a single they were replaced by Webex and Teams. European Medicines Agency’s (EMA) issue can help a sponsor enormously and (Secure Zoom meeting are to be added registration of the RNA interference accelerate the development cycle. Before this year.) And remarkably, by 31 December, (RNAi) therapy Leqvio (inclisiran) for COVID-19, it was conceivable that a small the US Food and Drug Administration cardiovascular disease. -

The Covid–19 Pandemic and Haemoglobin Disorders
THE COVID–19 PANDEMIC AND HAEMOGLOBIN DISORDERS VACCINATIONS & THERAPEUTIC DRUGS An Informational Guide from the Thalassaemia International Federation (TIF) Prepared by: Dr Androulla Eleftheriou, Executive Director, TIF Last Updated: 12 May 2020 VACCINATIONS & THERAPEUTIC DRUGS Introduction It is important to note that there are currently no FDA1 or EMA2-approved or even recommended agents for the treatment of the novel coronavirus (COVID-19), for which the World Health Organization (WHO) declared as pandemic on Wednesday 11th of March 2020. Any agent being used at this time is being administered in an experimental setting under controlled conditions. Thalassaemia International Federation (TIF) has made an effort to compile a list of studies/clinical trials for treatment and vaccines, which is by no means exhaustive as this situation is extremely labile and research in this area is dramatically intensified. New information is anticipated to be added to this guide which is prepared exclusively for TIF’s global thalassemia community. The viral genome was mapped very soon as rom early January 2020 and shared globally. In February 2020, the WHO published an overview of the potential therapeutic candidates for the treatment of COVID-19. The document outlines 76 regimens that have been proposed (as of February 17, 2020) for the treatment of patients infected with the virus. Thirty-eight of these candidates are in the preclinical state with minimal information available on their proposed mechanism, uses, doses routes, or planned trials. Sixteen of the remaining regimens contain an interferon-based product. The rest include a variety of antimicrobials, corticosteroids, convalescent plasma, and biologics. The Director-General of the WHO, Mr Tedros Adhanom, stated on the 10th of April 2020, that more than 70 countries have joined WHO’s trial to accelerate research on effective treatments and 20 Institutions and companies ‘are racing to develop a vaccine’. -

Amgen 2008 Annual Report and Financial Summary Pioneering Science Delivers Vital Medicines
Amgen 2008 Annual Report and Financial Summary Pioneering science delivers vital medicines About Amgen Products Amgen discovers, develops, manufactures Aranesp® (darbepoetin alfa) and delivers innovative human therapeutics. ® A biotechnology pioneer since 1980, Amgen Enbrel (etanercept) was one of the fi rst companies to realize ® the new science’s promise by bringing EPOGEN (Epoetin alfa) safe and effective medicines from the lab Neulasta® (pegfi lgrastim) to the manufacturing plant to patients. NEUPOGEN® (Filgrastim) Amgen therapeutics have changed the practice of medicine, helping millions of Nplate® (romiplostim) people around the world in the fi ght against ® cancer, kidney disease, rheumatoid Sensipar (cinacalcet) arthritis and other serious illnesses, and so Vectibix® (panitumumab) far, more than 15 million patients worldwide have been treated with Amgen products. With a broad and deep pipeline of potential new medicines, Amgen remains committed to advancing science to dramatically improve people’s lives. 0405 06 07 08 0405 06 07 08 0405 06 07 08 0405 06 07 08 Total revenues “Adjusted” earnings Cash fl ow from “Adjusted” research and ($ in millions) per share (EPS)* operations development (R&D) expenses* (Diluted) ($ in millions) ($ in millions) 2008 $15,003 2008 $4.55 2008 $5,988 2008 $2,910 2007 14,771 2007 4.29 2007 5,401 2007 3,064 2006 14,268 2006 3.90 2006 5,389 2006 3,191 2005 12,430 2005 3.20 2005 4,911 2005 2,302 2004 10,550 2004 2.40 2004 3,697 2004 1,996 * “ Adjusted” EPS and “adjusted” R&D expenses are non-GAAP fi nancial measures. See page 8 for reconciliations of these non-GAAP fi nancial measures to U.S.