The Embodied Mind in Sleep and Dreaming: a Theoretical Framework and an Empirical Study of Sleep, Dreams and Memory in Meditators and Controls
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phenomenology of Embodied Dreamwork with Puerto Rican Women: a Dissertation Lourdes F
Lesley University DigitalCommons@Lesley Graduate School of Arts and Social Sciences Expressive Therapies Dissertations (GSASS) 2010 Phenomenology of Embodied Dreamwork with Puerto Rican Women: A Dissertation Lourdes F. Brache-Tabar Lesley University Follow this and additional works at: https://digitalcommons.lesley.edu/expressive_dissertations Part of the Art Therapy Commons, Latin American Studies Commons, and the Psychology Commons Recommended Citation Brache-Tabar, Lourdes F., "Phenomenology of Embodied Dreamwork with Puerto Rican Women: A Dissertation" (2010). Expressive Therapies Dissertations. 46. https://digitalcommons.lesley.edu/expressive_dissertations/46 This Dissertation is brought to you for free and open access by the Graduate School of Arts and Social Sciences (GSASS) at DigitalCommons@Lesley. It has been accepted for inclusion in Expressive Therapies Dissertations by an authorized administrator of DigitalCommons@Lesley. For more information, please contact [email protected]. 1 Phenomenology of Embodied Dreamwork with Puerto Rican Women A DISSERTATION (submitted by) Lourdes F. Brache-Tabar In partial fulfillment of the requirements for the degree of Doctor of Philosophy LESLEY UNIVERSITY May 2010 2 3 STATEMENT BY AUTHOR This dissertation has been submitted in partial fulfillment of requirements for an advanced degree at Lesley University and is deposited in the University Library to be made available to borrowers under rules of the Library. Brief quotations from this dissertation are allowable without special permission, provided that accurate acknowledgment of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in his or her judgment the proposed use of the material is in the interests of scholarship. -

A Philosophy of the Dreaming Mind
Dream Pluralism: A Philosophy of the Dreaming Mind By Melanie Rosen A THESIS SUBMITTED TO MACQUARIE UNIVERSITY FOR THE DEGREE OF DOCTOR OF PHILOSOPHY DEPARTMENT OF COGNITIVE SCIENCE, FACULTY OF HUMAN SCIENCE MACQUARIE UNIVERSITY, NSW 2109, AUSTRALIA JULY 2012 Table of Contents Abstract 9 Declaration 11 Acknowledgements 13 Introduction 15 Part 1: Dream Pluralism 25 Chapter 1: The Empirical Study of Dreams: Discoveries and Disputes 27 1.1 Stages of sleep 29 1.1.1 NREM Sleep 30 1.1.2 REM Sleep 32 1.1.3 The Scanning Hypothesis: an attempt to correlate eye movements with dream reports 33 1.2 Dream reports 35 1.2.1 The benefits of lab-based research 36 1.2.2 The benefits of home-based research 38 1.3 Measuring the physiology of the sleeping brain and body 41 1.3.1 Physiological measures: pros and cons 42 1.4 Cognitive and neural features of sleep 48 1.5 Lucid dreamers in the dream lab 55 Conclusion 59 1 Chapter 2: Bizarreness and Metacognition in Dreams: the Pluralist View of Content and Cognition 61 2.1 A pluralistic account of dream content 62 2.1.1 Bizarre and incoherent dreams 63 2.1.2 Dreams are not particularly bizarre 66 2.1.3 Explanations of the conflicting results 69 2.1.4 Dreams vs. fantasy reports 72 2.2 Cognition in dreams: deficient or equivalent? 80 2.2.1 What is metacognition? 80 2.2.2 Metacognition in dreams 83 Conclusion 97 Chapter 3: Rethinking the Received View: Anti-Experience and Narrative Fabrication 99 3.1 Malcolm on dreaming 101 3.1.1 Dreams and verification 102 3.1.2 Evidence against Malcolm 109 3.2 Metaphysical anti-experience theses 115 3.2.1 The cassette view 115 3.2.2 Arguments against the cassette view 118 3.2.3 Consciousness requires recognition or clout 120 3.3 Narrative fabrication in dream reports 122 3.3.1 Rationalisation of strange content 123 3.3.2 Confabulation and memory loss 127 3.3.3 Altered states of consciousness and what it’s like to be a bat. -

Zerohack Zer0pwn Youranonnews Yevgeniy Anikin Yes Men
Zerohack Zer0Pwn YourAnonNews Yevgeniy Anikin Yes Men YamaTough Xtreme x-Leader xenu xen0nymous www.oem.com.mx www.nytimes.com/pages/world/asia/index.html www.informador.com.mx www.futuregov.asia www.cronica.com.mx www.asiapacificsecuritymagazine.com Worm Wolfy Withdrawal* WillyFoReal Wikileaks IRC 88.80.16.13/9999 IRC Channel WikiLeaks WiiSpellWhy whitekidney Wells Fargo weed WallRoad w0rmware Vulnerability Vladislav Khorokhorin Visa Inc. Virus Virgin Islands "Viewpointe Archive Services, LLC" Versability Verizon Venezuela Vegas Vatican City USB US Trust US Bankcorp Uruguay Uran0n unusedcrayon United Kingdom UnicormCr3w unfittoprint unelected.org UndisclosedAnon Ukraine UGNazi ua_musti_1905 U.S. Bankcorp TYLER Turkey trosec113 Trojan Horse Trojan Trivette TriCk Tribalzer0 Transnistria transaction Traitor traffic court Tradecraft Trade Secrets "Total System Services, Inc." Topiary Top Secret Tom Stracener TibitXimer Thumb Drive Thomson Reuters TheWikiBoat thepeoplescause the_infecti0n The Unknowns The UnderTaker The Syrian electronic army The Jokerhack Thailand ThaCosmo th3j35t3r testeux1 TEST Telecomix TehWongZ Teddy Bigglesworth TeaMp0isoN TeamHav0k Team Ghost Shell Team Digi7al tdl4 taxes TARP tango down Tampa Tammy Shapiro Taiwan Tabu T0x1c t0wN T.A.R.P. Syrian Electronic Army syndiv Symantec Corporation Switzerland Swingers Club SWIFT Sweden Swan SwaggSec Swagg Security "SunGard Data Systems, Inc." Stuxnet Stringer Streamroller Stole* Sterlok SteelAnne st0rm SQLi Spyware Spying Spydevilz Spy Camera Sposed Spook Spoofing Splendide -

Awake in the Dark: Imageless Lucid Dreaming Linda L. Magallon San Jose, California Most Dream Research, Interpretation Methodolo
Lucidity Letter June, 1987, Vol. 6, No. 1 Awake in the Dark: Imageless Lucid Dreaming Linda L. Magallon San Jose, California Most dream research, interpretation methodology and reports of dreaming phenomena presuppose that a dream consists of visual impressions. Even the term LUCIDITY evokes the vividness and clarity of dream imagery. Yet, there is ample experiential evidence to warrant a rethinking of this assumption. Imageless lucidity can and does occur at all levels of dreaming. Entry via Hypnagogia As the dreamer drifts into dreaming through lucid hypnagogia, watching the imagery flicker and metamorphose, she or he may encounter a "blank" period just before the dream scene appears. In this state, there is no sensation but rather the general impression that the dream is "taking a breath" before forming a landscape in the dreamer's mind. The Initial Awakening State This is the lucid equivalent of the false awakening state, reported by such notables as Dr. van Eeden and Oliver Fox. The dreamer may become aware of auditory stimuli unrelated to waking sounds. If tactile sensation is retained, the dreamer can 1 Lucidity Letter June, 1987, Vol. 6, No. 1 eventually experience a sense of duality or bilocation as he or she moves into deeper dreaming. None of this need be accompanied by images. An excerpt from my own dream journal provides an example of this state, experienced as an imageless dream: (When the hypnagogic images fade,) I become aware of a continuous conversation, which I assume means I have reached a telepathic level. I concentrate to determine the quality of this level in order to conjure it up in the waking state. -

09 Supplement.Indd
I J o D R Abstracts of the 33th Annual Conference of the International Association for the Study of Dreams June 24 - June 28, 2016 Kerkrade, The Netherlands Content This supplement of the International Journal of Dream Research includes the abstracts of presenters who gave consent to the publishing. The abstracts are categorized into thematic groups and within the category sorted according to the last name of the fi rst presenter. Affi liations are included only for the fi rst author. A name register at the end is also provided. can be our guides in this stupendous process of birthing Contents: a new civilization, free from the indescribable cruelties and power struggles which have polluted the past and still des- 1. Keynotes ecrate the present. In ancient Egypt and Greece, in China 2. Morning Dream Groups and South America, in Sumer, Persia and Babylon and in Hebrew culture, this visionary guidance was welcomed and 3. Workshops honoured. In contemporary indigenous cultures it still is and 4. Clinical Topics they have much to teach us. The message of the visionary dream is to bring the human community into harmonious re- 5. Religion/Spiritual/Culture/Arts lationship with the hidden laws governing the cosmos. Our 6. Education/Other Topics culture has become disconnected from the invisible dimen- sion of reality, ignoring the presence of these hidden laws. 7. PSI Dreaming Consequently, it has become impoverished and ailing, like 8. Lucid Dreaming the Old King of Alchemy. 9. Research/Theory Fortunately, in the last century, there was a great Dreamer, a psychiatrist called C.G. -

DREAMS HEAL 2 How Dreams Heal Introduction When We Sleep, Our
DREAMS HEAL 2 How Dreams Heal Introduction When we sleep, our brains do not turn off. Instead they process and problem-solve in our dreams. The images, environments, and stories of our dreams often tackle situations we feel unequipped to face in our waking lives. The question remains, however, are these dream images consequential in the mitigation of the painful symptoms experienced as a result of emotional trauma? Dargert (2016) proposes that while neuroscience explores the relationship of brain to mind, the psyche is for the most part unexamined. For the purposes of this paper I will explore the interface between Depth and Archetypal psychology theories and science with an eye toward how they might be integrated to promote a new vision of how dreams can be used to heal trauma symptoms. Embodied Imagination (EI), a kind of guided dream work, is concerned with the direct effect of imagination on the mind, body and spirit, incorporating both archetypal psychology and dream science. Therefore, an exploration of the author’s understanding and experience of the healing properties of EI will also be explored. The Science of Dreaming Despite claims to the contrary regarding rapid eye movement (REM) sleep, dreaming has no clear biological marker. In research cited by Pagel (2014), REM sleep occurred without dreaming and vice-versa. He maintains that it is still unclear whether any special relationship exists between REM sleep and dreaming, stating that “there are electrophysiological, DREAMS HEAL 3 neurochemical, anatomical and physiological systems of dreaming, with each of these systems affected by a wide variety of medical, psychological, sleep, and social variables” (p.xix). -

The Nature and Function of the Hypnagogic State Thesis Submitted
HYPNAGOGIA The Nature and Function of the Hypnagogic State by Andreas Mavromatis Thesis submitted to the Department of Psychology, Brunel University, for the degree of Doctor of Philosophy January 1983 V01-1 PIS 4: r.:: ; D Eiz. -D Dream caused by the flight of a bee around a pomegranate one second before waking up 1944 Oil()" canvas. ;1x 41 Thyssen-Bornemis_a Collection. Lugano SalvadorDeli -' i 1 y1 \i ý;,, ý. ý,ý, 4' l ! ,,.: . >" ý -d Rupp- a All 4ý Vic All CONTENTS Abstract 3 Acknowledgements 5 Preface 6 - PART ONE - PHENOMENOLOGY 1. Introduction 8 2. Historical background and incidence 12 3. Methods and procedures of investigation 19 4. Sensori-motor phenomena and systems of classification 25 5. Physiological correlates 64 6" Problems of definition and the stages of the hypnagogic state 73 7. Cognitive-affective characteristics 83 Summary and Conclusions of Part One 131 - PART TWO - HYPNAGOGIA AND ITS RELATIONSHIP TO OTHER STATES, PROCESSES, AND EXPERIENCES Introduction 137 Be Hypnosis 139 9. Dreams 150 10. Meditation 183 11. Psi 212 12. Schizophrenia 265 13. Creativity 310 14. Other areas of experience 374 Summary and Conclusions of Part Two 388 - PART THREE - GRAIN MECHANISMS AND FUNCTION OF HYPNAGOGIA Introduction 394 15. Cerebral correlates of hypnagogic visions 395 16. Cerebral correlates of hypnagogic mentation 420 17. The old versus the new brain 434 18. The loosening of ego boundaries 460 19. The function of hypnagogia 474 20. The significance of hypnagogia 492 Appendix 510 Bibliography 519 ANDREAS MAVROMATIS Ph. D. Psychology, Brunel University, 1983. - HYPNAGOGIA - The Nature and Function of the Hypnagogic State ABSTRACT An analysis of the hypnagogic state (hypnagogia) leads to the conclusion that, far from being a simple phase of sleep, this state or process is a central phenomenon characterized by a constellation of psychological features which emerge as a function of the hypnagogic subject's loosening of ego boundaries (LEB) and are correlated with activities of subcortical structures. -

The Nightmare Free
FREE THE NIGHTMARE PDF lars Kepler | 608 pages | 03 Jan 2013 | HarperCollins Publishers | 9780007414505 | English | London, United Kingdom The Nightmare - Wikipedia The Nightmare By Henry Fuseli. Regarded as one of the The Nightmare Paintings Ever. For the meaning of other celebrated masterpieces, please see: Famous Paintings Analyzed One of the most innovative Romantic artists of his day, the Swiss-born Johann Heinrich Henry Fuseli - son of the The Nightmare Johann Caspar Fussli - developed an early talent for drawing before moving to London The Nightmare at the age of Here, encouraged by Joshua Reynolds who was shortly to be elected the first president of the newly formed Royal Academy of ArtsFuseli took up painting. This led him to spend most of the s in Italy, studying the figure painting of Michelangelo which became a major influence on his art. Other influences included 16th-century Mannerism and literary sources, notably Shakespeare. Later appointed a professor of painting at the Royal Academy, he became one of the best English painters of the eighteenth century and was buried in St Paul's Cathedral. Like his The Nightmare contemporary William BlakeFuseli's strength as a painter lies in his imaginative intensity, and The Nightmare - which he sold for 20 guineas - remains his greatest and most baffling masterpiece. Overlooked after his death, Fuseli was 'rediscovered' by 20th-century Expressionists and Surrealists who greatly admired his creativity. Painted shortly after his return from Italy, The Nightmare was first shown to the public in The Nightmare the annual exhibition of the Royal Academy. An instant success, it established Fuseli's reputation as one of the most creative artists in London. -

Astral Travelling
Out-of-Body Experiences Part 4 Astral Travelling Alfred Ballabene [email protected] [email protected] [email protected] Table of Contents 1. Is Astral Travelling the same as Lucid Dreaming?......................................................... 1 2. Basic Knowledge about Astral Travelling ....................................................................... 6 Does Astral Travelling Affect Night's Sleep? .................................................................. 7 Perception, emotions and way of thinking ...................................................................... 8 Differences between OBEs close to and far from the Body ........................................... 9 Commercialisation and trivialisation ............................................................................. 10 Deeper sense of astral travelling ..................................................................................... 11 3. Examples for Verification ................................................................................................. 12 4. The Findings of Sleep Research ....................................................................................... 15 5. Methods to increase Lucidity .......................................................................................... 16 6. General Advices on OBEing ............................................................................................ 18 7. Spontaneous Astral Travelling ....................................................................................... -

By JANET GAIL PATTERSON a Dissertation Submitted in Partial
THE BODY DREAMING: ENGAGING THE DREAM’S DISTURBING IMAGE by JANET GAIL PATTERSON A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY IN PSYCHOLOGY MERIDIAN UNIVERSITY 2013 THE BODY DREAMING: ENGAGING THE DREAM’S DISTURBING IMAGE by JANET GAIL PATTERSON A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY IN PSYCHOLOGY MERIDIAN UNIVERSITY 2013 This dissertation has been accepted for the faculty of Meridian University by: _________________________________________ Aftab Omer, Ph.D. Dissertation Advisor __________________________________________ Melissa Schwartz, Ph.D. Dissertation Chair __________________________________________ Shoshana Fershtman, Ph.D. Dissertation Committee Member Nightmares fill with light like a holiday. Men and angels speak one language. The elusive ones finally meet. The essence and evolving forms run to meet each other like children to their father and mother. Good and evil, dead and alive, everything blooms from one natural stem. --Rumi from "The Elusive Ones" Night and Sleep iv ABSTRACT THE BODY DREAMING: ENGAGING THE DREAM'S DISTURBING IMAGE by Janet Patterson This study posed the Research Problem: In what ways does working affectively and somatically with disturbing dream images affect adaptive identity? It was hypothesized that working with disturbing dream images somatically and affectively would allow the dreamer to experience negative affects, thus broadening the experience and acceptance of their own multiplicity. The theory-in-practice was Imaginal Transformation Praxis (ITP). The literature review selects works from psychological, psychobiological, popular, and cross-cultural approaches to dreams; Imaginal Approaches to dreams, including somatic dream work, psychological multiplicity, and affect theory; and disturbing imagery. -
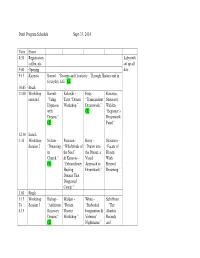
Draft Program Schedule Sept. 13, 2014 Time Event 8:30 Registration
Draft Program Schedule Sept. 13, 2014 Time Event 8:30 Registration, Labyrinth coffee, etc. set up all 9:00 Opening day. 9:15 Keynote Barrett – “Dreams and Creativity—Through History and in Everyday Life” CE 10:45 Break 11:00 Workshop Barrett - Kolinski – Hoss - Kanavos, session 1 “Using Tarot “Dream “Transcendent Strisower, Hypnosis Workshop.” Dreamwork.” Walden- with CE “Beginner’s Dreams.” Dreamwork CE Panel.” 12:30 Lunch 1:30 Workshop Nelson - Paseman - Berry - Strisower - Session 2 “Dreaming “Whirlwinds of “Drawn into “Facets of in the Soul” the Dream, a Dream Church.” & Kanavos - Visual Work CE “Extraordinary Approach to Beyond Healing Dreamwork.” Dreaming.” Dreams That Diagnosed Cancer.” 3:00 Break 3:15 Workshop Bishop - Walden - White - Schiffman To Session 3 “Addiction “Dream “Embodied – “The 4:15 Recovery Theater Imagination & Akashic Dreams.” Workshop.” Veterans’ Records CE Nightmares.” and CE Dreams.” 4:15 Presenter’s Reception and sales. Meet and talk with presenters and see to material for sale by presenters & attendees. 5:00 Alphabetical list 1. Deirdre Barrett, PhD: “Using Hypnosis to Work with Your Dreams.” 2. Walter Berry, M.A.: “Drawn into the Dream, a Visual Approach to Dreamwork.” 3. Barbara Bishop, MFT: “Making Use of Dreams in Addiction Recovery.” 4. Robert Hoss, MS: “Transcendent Dreamwork.” 5. Athena Kolinski, M.A.: “Open the Gateway to your Inner Wisdom: A Tarot Dream Interpretation Workshop.” 6. Rev. Geoff Nelson, D.Min: “Dreaming in Church.” 7. Richard Paseman, Ed. D.: “Whirlwinds of the Soul: Dreams in a Desert Place” 8. Kathleen O’Keefe-Kanavos: “Extraordinary Healing Dreams That Diagnosed Cancer.” 9. Barbara Schiffman, “The Akashic Records: Accessing Higher Consciousness Dimensions for Creativity Enhancement, Dream Exploration & Personal Evolution.” 10. -

Externalism in Philosophy of Perception and Argument(S) from Dreaming
Externalism in Philosophy of Perception and Argument(s) from Dreaming by Doğan Erişen B.A., Bilkent University, 2015 Thesis Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Arts in the Department of Philosophy Faculty of Arts and Sciences © Doğan Erişen 2019 SIMON FRASER UNIVERSITY Spring 2019 Copyright in this work rests with the author. Please ensure that any reproduction or re-use is done in accordance with the relevant national copyright legislation. Approval Name: Doğan Erişen Degree: Master of Arts Title: Externalism in Philosophy of Perception and Argument(s) from Dreaming Examining Committee: Chair: Holly Andersen Associate Professor Kathleen Akins Senior Supervisor Professor Martin Hahn Supervisor Associate Professor Murat Aydede External Examiner Professor Department of Philosophy University of British Columbia Date Defended/Approved: April 11, 2019 ii Abstract A recurrent pattern of debate between the proponents of internalism and externalism over mental phenomena is as follows: externalists pick a target mental phenomenon, say, visual perception, and argue that it has the characteristics it has because of a property that is not possessed internally. Internalists, in return, substitute an analogue mental phenomenon, one that putatively suits their position, to argue that it shows every characteristic that the original target phenomenon shows, thus the allegedly crucial external property plays no ineliminable role. Within these debates a particular analogue phenomenon frequently appears: dreaming. In what follows, I discuss the ways in which externalism comes under dispute through dream phenomena. I then investigate the scientific literature to evaluate whether the way dreaming is conceived by internalists is substantiated by the available body of evidence.