Size Variations of Flowering Characters in Arum Maculatum (Araceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Liliaceae S.L. (Lily Family)
Liliaceae s.l. (Lily family) Photo: Ben Legler Photo: Hannah Marx Photo: Hannah Marx Lilium columbianum Xerophyllum tenax Trillium ovatum Liliaceae s.l. (Lily family) Photo: Yaowu Yuan Fritillaria lanceolata Ref.1 Textbook DVD KRR&DLN Erythronium americanum Allium vineale Liliaceae s.l. (Lily family) Herbs; Ref.2 Stems often modified as underground rhizomes, corms, or bulbs; Flowers actinomorphic; 3 sepals and 3 petals or 6 tepals, 6 stamens, 3 carpels, ovary superior (or inferior). Tulipa gesneriana Liliaceae s.l. (Lily family) “Liliaceae” s.l. (sensu lato: “in the broad sense”) - Lily family; 288 genera/4950 species, including Lilium, Allium, Trillium, Tulipa; This family is treated in a very broad sense in this class, as in the Flora of the Pacific Northwest. The “Liliaceae” s.l. taught in this class is not monophyletic. It is apparent now that the family should be treated in a narrower sense and some of the members should form their own families. Judd et al. recognize 15+ families: Agavaceae, Alliaceae, Amarylidaceae, Asparagaceae, Asphodelaceae, Colchicaceae, Dracaenaceae (Nolinaceae), Hyacinthaceae, Liliaceae, Melanthiaceae, Ruscaceae, Smilacaceae, Themidaceae, Trilliaceae, Uvulariaceae and more!!! (see web reading “Consider the Lilies”) Iridaceae (Iris family) Photo: Hannah Marx Photo: Hannah Marx Iris pseudacorus Iridaceae (Iris family) Photo: Yaowu Yuan Photo: Yaowu Yuan Sisyrinchium douglasii Sisyrinchium sp. Iridaceae (Iris family) Iridaceae - 78 genera/1750 species, Including Iris, Gladiolus, Sisyrinchium. Herbs, aquatic or terrestrial; Underground stems as rhizomes, bulbs, or corms; Leaves alternate, 2-ranked and equitant Ref.3 (oriented edgewise to the stem; Gladiolus italicus Flowers actinomorphic or zygomorphic; 3 sepals and 3 petals or 6 tepals; Stamens 3; Ovary of 3 fused carpels, inferior. -

Introduction to Common Native & Invasive Freshwater Plants in Alaska
Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska Cover photographs by (top to bottom, left to right): Tara Chestnut/Hannah E. Anderson, Jamie Fenneman, Vanessa Morgan, Dana Visalli, Jamie Fenneman, Lynda K. Moore and Denny Lassuy. Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska This document is based on An Aquatic Plant Identification Manual for Washington’s Freshwater Plants, which was modified with permission from the Washington State Department of Ecology, by the Center for Lakes and Reservoirs at Portland State University for Alaska Department of Fish and Game US Fish & Wildlife Service - Coastal Program US Fish & Wildlife Service - Aquatic Invasive Species Program December 2009 TABLE OF CONTENTS TABLE OF CONTENTS Acknowledgments ............................................................................ x Introduction Overview ............................................................................. xvi How to Use This Manual .................................................... xvi Categories of Special Interest Imperiled, Rare and Uncommon Aquatic Species ..................... xx Indigenous Peoples Use of Aquatic Plants .............................. xxi Invasive Aquatic Plants Impacts ................................................................................. xxi Vectors ................................................................................. xxii Prevention Tips .................................................... xxii Early Detection and Reporting -

Bibliography
Bibliography Albre, J., Quilichini, A. & Gibernau, M., 2003. Pollination ecology of Arum italicum (Araceae). Botanical Journal of the Linnean Society, 141(2), 205–214. Allen, G. 1881. The Evolutionist at Large, Chatto & Windus. Available at: http://archive.org/details/ evolutionistatl00allegoog [accessed 19 August, 2012]. Allen, G. 1897. The Evolution of the Idea of God: An Inquiry into the Origins of Religion, H. Holt and company. Available at: http://archive.org/details/evolutionideago00allegoog [accessed 19 August, 2012]. Anon., 1526. The Grete Herball. Translated from French by Peter Treveris: London. Anon., 1833. History of Vegetable Substances Used in the Arts, in Domestic Economy, and for the Food of Man, Boston, Lilly, Wait, Colman and Holden. Available at: http://archive.org/details/ historyofvegetab02bostiala [accessed 12 January, 2013]. Anon., 1838. Stone quarries and beyond. The Penny Magazine. Available at: http:// quarriesandbeyond.org/articles_and_books/isle_of_portland.html [accessed 12 January, 2013]. Anon., 1861. The Physicians of Myddvai; Meddygon Myddvai, or The Medical Practice of the Celebrated Riwallon and his Sons, of Myddvai, in Caermarthenshire, Physicians to Rhys Gryg, Lord of Dynevor and Ystrad Towy, about the Middle of the Thirteenth Century, Llandovery: D.J. Roderic, London, Longman & Co. Available at: http://archive.org/details/ physiciansofmydd00llan [accessed 12 January, 2013]. Anon., 2004. Arum maculatum (for advanced foragers only). BushcraftUK. Available at: http:// www.bushcraftuk.com/forum/showthread.php?t=3862 [accessed 12 January, 2013]. Anon., 2008. Herbal Medicine Diploma Course. A Brief History of Herbalism. Lesson 1. Anon., 2012. Wihtburh [Withburga]. Wikipedia, the free encyclopedia. Available at: https:// en.wikipedia.org/w/index.php?title=Wihtburh&oldid =530340470 [accessed 12 January, 2013]. -

Size Variations of Flowering Characters in Arum Italicum (Araceae)
M. GIBERNAU,]. ALBRE, 2008 101 Size Variations of Flowering Characters in Arum italicum (Araceae) Marc Gibernau· and Jerome Albre Universite Paul Sabatier Laboratoire d'Evolution & Diversite Biologique (UMR 5174) Bat.4R3-B2 31062 Toulouse cedex 9 France *e-mail: [email protected] ABSTRACT INTRODUCTION In Arum, bigger individuals should An extreme form of flowering character proportionally invest more in the female variations according to the size is gender function (number or weight of female modification, which occurs in several flowers) than the male. The aim of this species of Arisaema (Clay, 1993). Individ paper is to quantify variations in repro ual plant gender changes from pure male, ductive characters (size of the spadix when small, to monoecious (A. dracon parts, number of inflorescences) in rela tium) or pure female (A. ringens) when tion to plant and inflorescence sizes. The large (Gusman & Gusman, 2003). This appendix represents 44% of the spadix gender change is reversible, damaged length. The female zone length represents female individuals will flower as male the 16.5% of the spadix length and is much following year (Lovett Doust & Cavers, longer than the male zone (6%). Moreover 1982). These changes are related to change these three spadix zones increase with in plant size and are explained by the plant vigour indicating an increasing size-advantage model. The size-advantage investment into reproduction and pollina model postulates a sex change when an tor attraction. It appears that the length of increase in body size is related to differen appendix increased proportionally more tial abilities to produce or sire offspring than the lengths of the fertile zones. -

Indigenous Plants of Bendigo
Produced by Indigenous Plants of Bendigo Indigenous Plants of Bendigo PMS 1807 RED PMS 432 GREY PMS 142 GOLD A Gardener’s Guide to Growing and Protecting Local Plants 3rd Edition 9 © Copyright City of Greater Bendigo and Bendigo Native Plant Group Inc. This work is Copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission from the City of Greater Bendigo. First Published 2004 Second Edition 2007 Third Edition 2013 Printed by Bendigo Modern Press: www.bmp.com.au This book is also available on the City of Greater Bendigo website: www.bendigo.vic.gov.au Printed on 100% recycled paper. Disclaimer “The information contained in this publication is of a general nature only. This publication is not intended to provide a definitive analysis, or discussion, on each issue canvassed. While the Committee/Council believes the information contained herein is correct, it does not accept any liability whatsoever/howsoever arising from reliance on this publication. Therefore, readers should make their own enquiries, and conduct their own investigations, concerning every issue canvassed herein.” Front cover - Clockwise from centre top: Bendigo Wax-flower (Pam Sheean), Hoary Sunray (Marilyn Sprague), Red Ironbark (Pam Sheean), Green Mallee (Anthony Sheean), Whirrakee Wattle (Anthony Sheean). Table of contents Acknowledgements ...............................................2 Foreword..........................................................3 Introduction.......................................................4 -

The Evolution of Pollinator–Plant Interaction Types in the Araceae
BRIEF COMMUNICATION doi:10.1111/evo.12318 THE EVOLUTION OF POLLINATOR–PLANT INTERACTION TYPES IN THE ARACEAE Marion Chartier,1,2 Marc Gibernau,3 and Susanne S. Renner4 1Department of Structural and Functional Botany, University of Vienna, 1030 Vienna, Austria 2E-mail: [email protected] 3Centre National de Recherche Scientifique, Ecologie des Foretsˆ de Guyane, 97379 Kourou, France 4Department of Biology, University of Munich, 80638 Munich, Germany Received August 6, 2013 Accepted November 17, 2013 Most plant–pollinator interactions are mutualistic, involving rewards provided by flowers or inflorescences to pollinators. An- tagonistic plant–pollinator interactions, in which flowers offer no rewards, are rare and concentrated in a few families including Araceae. In the latter, they involve trapping of pollinators, which are released loaded with pollen but unrewarded. To understand the evolution of such systems, we compiled data on the pollinators and types of interactions, and coded 21 characters, including interaction type, pollinator order, and 19 floral traits. A phylogenetic framework comes from a matrix of plastid and new nuclear DNA sequences for 135 species from 119 genera (5342 nucleotides). The ancestral pollination interaction in Araceae was recon- structed as probably rewarding albeit with low confidence because information is available for only 56 of the 120–130 genera. Bayesian stochastic trait mapping showed that spadix zonation, presence of an appendix, and flower sexuality were correlated with pollination interaction type. In the Araceae, having unisexual flowers appears to have provided the morphological precon- dition for the evolution of traps. Compared with the frequency of shifts between deceptive and rewarding pollination systems in orchids, our results indicate less lability in the Araceae, probably because of morphologically and sexually more specialized inflorescences. -

Improving the Quality of Ornamental Bulbous with Plant Growth-Promoting Rhizobacteria (Pgpr)
ISSN (Online): 2455-3662 EPRA International Journal of Multidisciplinary Research (IJMR) - Peer Reviewed Journal Volume: 7 | Issue: 5 | May 2021|| Journal DOI: 10.36713/epra2013 || SJIF Impact Factor 2021: 8.047 || ISI Value: 1.188 IMPROVING THE QUALITY OF ORNAMENTAL BULBOUS WITH PLANT GROWTH-PROMOTING RHIZOBACTERIA (PGPR) Domenico Prisa1 , Alessandra Benati2 1CREA Research Centre for Vegetable and Ornamental Crops, Council for Agricultural Research and Economics, Via dei Fiori 8, 51012 Pescia, PT, Italy 2Associazione P.A.C.M.E. Le Tribù della Terra ONG 1Corresponding author: Domenico Prisa Article DOI: https://doi.org/10.36713/epra7029 DOI No: 10.36713/epra7029 ABSTRACT The aim of this work was to use Plant growth promoting rhizobacteria (PGPR) for the improvement of cultivation and agronomic and pathogen protection characteristics of ornamental bulbous plants such as Tulip (fam. Liliacee), Iris (fam. Iridacee), Freesia (fam. Iridacee) and Narcissus (fam. Amarillidacee). The experiments, started in November 2020, were conducted in the greenhouses of CREA-OF in Pescia (Pt), Tuscany, Italy. The experimental groups were: i) group control irrigated with water and substrate previously fertilized; ii) group with Effective microorganisms irrigated with water and substrate previously fertilized; iii) group with beneficial bacteria (TNC Bactorrs13) irrigated with water and substrate previously fertilized; iv) group with beneficial bacteria (Tarantula powder Advanced nutrients ) irrigated with water and substrate previously fertilized. The trial showed a significant improvement in the agronomic parameters analysed on plants obtained from Narcissus, Iris, Tulip and Freesia bulbs treated with microorganisms. In particular, there was an increase in plant height, vegetative and root weight, bulb weight and diameter, and flower duration. -

Italian Arum Flyer
PUBLIC ANNOUNCEMENT- WESTERN INVASIVES NETWORK Revised April, 2020 847 NW Monroe Ave. Corvallis, OR 97330 (541) 910-8769 Photo: Travis Johnson 1Photo: Liz West************ NOXIOUS WEED ALERT************ Italian Arum Italian arum (Arum italicum), also known as Digging and disposing of the small “orange candleflower” and “Italian lords and underground corms in the garbage can help. ladies,” is a non-native perennial that was However, manual removal is only introduced as an ornamental plant. It has recommended on small patches, because if now naturalized and appears to be the plant is not completely eradicated, the spreading. Arum italicum is a woodland soil disturbance can increase its spread. If species and prefers moist, well-shaded you have it on your land, do not let it go to environments. It’s extremely difficult to seed. Wearing gloves, cut and bag all seed eradicate once it becomes established and heads, and dispose of them in the garbage. may spread from residential gardens into The variegated, exotic leaves and showy red fruits woodland areas. For more info on Italian arum: make it an attractive garden plant. Unfortunately, Western Oregon is the perfect environment for Why is it a problem? WA Noxious Weed Control Board unwanted invasions. (Photo: East Multnomah SWCD) This plant is problematic because of its ability Lower Hudson PRISM to form a dense cover in open sites. It can out-compete native plants and prevent their The Western Invasives Network encourages establishment. you to report Italian arum, in an effort to inform future management priorities across Italian arum is also toxic to humans and western Oregon. -

Environmental and Anthropogenic Pressures on Geophytes of Iran and the Possible Protection Strategies: a Review
International Journal of Horticultural Science and Technology Vol. 2, No. 2; December 2015, pp 111-132 Environmental and Anthropogenic Pressures on Geophytes of Iran and the Possible Protection Strategies: A Review Homayoun Farahmand1* and Farzad Nazari2 1. Department of Horticultural Science, Faculty of Agriculture, Shahid Bahonar University of Kerman, Kerman, Iran. 2. Department of Horticultural Science, College of Agriculture, University of Kurdistan, Sanandaj, Iran. )Received: 12 August 2015, Accepted: 3 October 2015( Abstract Ornamental geophytes (ornamental flower bulbs) are international and national heritage considering their contribution to people's life quality around the world. Iranian habitats support about 8000 species of flowering plants (belonging to 167 families and 100 genera) of which almost 1700 are endemic. Iran is a rich country in terms of distribution of bulbous plants. More than 200 species of bulbous species from different plant families naturally grow in Iran and play an important role in the colorful display of flowers in the plains, mountains, and forests. Unfortunately, some flower bulbs are at the risk of eradication in Iran due to some factors, including inappropriate herboviry and overgrazing, land use change, illegal bulb and flower harvesting, road construction, mining activities, drought, etc. The establishment of protected areas, efficient propagation methods such as micropropagation, gathering the species at the risk of extinction in Botanical Gardens and Research Centers, highlighting the decisive role of Non- governmental organizations (NGOs), and improving tourism are some approaches suggested for better conservation. Meanwhile, under the current situation, national and international protecting rules and regulations should be assigned and fulfilled to save this invaluable natural heritage. -
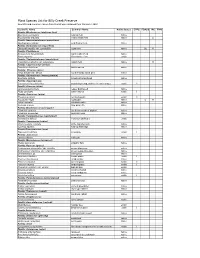
Plant Species List for Billy Creek Preserve Scientific and Common Names from This List Were Obtained from Wunderlin 2003
Plant Species List for Billy Creek Preserve Scientific and Common names from this list were obtained from Wunderlin 2003 Scientific Name Common Name Native Status EPPC FDACS IRC FNAI Family: Blechnaceae (midsorus fern) Blechnum serrulatum swamp fern native Woodwardia areolata netted chain fern native CI Family: Nephrolepidaceae (sword fern) Nephrolepis exaltata wild Boston fern native Family: Osmundaceae (royal fern) Osmunda regalis var. spectabilis royal fern native CE R Family: Pteridaceae Acrostichum danaeifolium giant leather fern native Pteris tripartita Giant brake fern exotic Family: Thelypteridaceae (marsh fern) Thelypteris palustris var. pubescens marsh fern native R Family: Cupressaceae (cedar) Taxodium distichum bald-cypress native Family: Pinaceae (pine) Pinus elliottii var. densa south Florida slash pine native Family: Alismataceae (water plantain) Sagittaria latifolia broadleaf arrowhead native Family: Asparagaceae Sansevieria hyacinthoides bowstring hemp, mother-in-law's tongue exotic II Family: Araceae (arum) Lemna aequinoctialis lesser duckweed native Pistia stratiotes water lettuce exotic I Family: Arecaceae (palm) Phoenix reclinata reclinata palm exotic II Roystonea regia royal palm native E R Sabal palmetto cabbage palm native Serenoa repens saw palmetto native Family: Bromeliaceae (pineapple) Tillandsia setacea southern needleaf airplant native Tillandsia usneoides spanish moss native Family: Commelinaceae (spiderwort) Commelina diffusa common dayflower exotic Family: Cyperaceae (sedge) Rhynchospora colorata white -

Italian Arum What Is Italian Arum ? Italian Arum, Arum Italicum, Is Also Known As Orange Candleflower, Cuckoo’S Pint, and Italian Lords-And-Ladies
February Invasive Plant Highlight Italian Arum What is Italian Arum? Italian Arum, Arum italicum, is also known as Orange Candleflower, Cuckoo’s Pint, and Italian Lords-and-Ladies. It is native to the Mediterranean region of Europe. It has been cultivated as an ornamental plant for traditional and shade gardens in the U.S. Italian arum is a perennial, herbaceous plant that grows from small pea-sized white bulbs. Many bulbs are produced during the growing season and they make it difficult to eradicate the plant. Italian Arum leaves Italian Arum is classified as a toxic weed. It is poisonous to humans if ingested and can cause an allergic reaction if touched without using gloves. Italian Arum can grow 12 to 18 inches. It blooms in the spring with white flowers that make bright orangish-red fruit. It spreads when its seed disperses by water or by birds consuming the berries. The plant invades riparian forest areas, threatening native plant diversity and also can cause increased erosion and slope instability along streambanks. Italian Arum berries Better Groundcovers Alumroot, Wild Ginger, Mayapple, Jack-in-the-pulpit, and Allegheny Spurge Please Remove It! If you have Italian Arum, please remove it before it spreads into other adjacent properties. Italian Arum emerges in the Fall and will stay green during mild winters making it easy to spot. Herbicides are not effective since death of foliage above ground does not indicate that the bulbs have died. To remove small patches with a trowel, dig down to root end (about 6 inches) and sift the soil to extract all the small bulbs. -

History and Current Status of Systematic Research with Araceae
HISTORY AND CURRENT STATUS OF SYSTEMATIC RESEARCH WITH ARACEAE Thomas B. Croat Missouri Botanical Garden P. O. Box 299 St. Louis, MO 63166 U.S.A. Note: This paper, originally published in Aroideana Vol. 21, pp. 26–145 in 1998, is periodically updated onto the IAS web page with current additions. Any mistakes, proposed changes, or new publications that deal with the systematics of Araceae should be brought to my attention. Mail to me at the address listed above, or e-mail me at [email protected]. Last revised November 2004 INTRODUCTION The history of systematic work with Araceae has been previously covered by Nicolson (1987b), and was the subject of a chapter in the Genera of Araceae by Mayo, Bogner & Boyce (1997) and in Curtis's Botanical Magazine new series (Mayo et al., 1995). In addition to covering many of the principal players in the field of aroid research, Nicolson's paper dealt with the evolution of family concepts and gave a comparison of the then current modern systems of classification. The papers by Mayo, Bogner and Boyce were more comprehensive in scope than that of Nicolson, but still did not cover in great detail many of the participants in Araceae research. In contrast, this paper will cover all systematic and floristic work that deals with Araceae, which is known to me. It will not, in general, deal with agronomic papers on Araceae such as the rich literature on taro and its cultivation, nor will it deal with smaller papers of a technical nature or those dealing with pollination biology.