Differentiation of Lard, Chicken Fat, Beef Fat and Mutton Fat by GCMS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chinese Express Ingredients and Allergen Information
Asian Ingredient and Allergen Information Item Name Ingredient Statement Allergen Statement Appetizers Chicken Egg Roll (Cabbage, Enriched Bleached Flour [{Wheat Flour, Niacin, Reduced Iron, Thiamine Mononitrate, Riboflavin, Folic Acid}, Calcium Propionate, Malted Barley Flour], Water, White Meat Chicken, Carrots, Onion, Textured Soy Flour, Celery, Enriched Durum Flour [Wheat Flour, Niacin, Ferrous Sulfate, Thiamine Mononitrate, Riboflavin, Folic Acid], Contains 2% or less of: Vegetable Oil [Soybean, Cottonseed, Corn, and/or Canola Oil], Salt, Autolyzed Yeast Extract, Modified Food Starch, Sugar, Garlic, Dried Whole Egg, Soy Sauce Powder [Soy Sauce {Wheat, Soybeans, Salt}, Maltodextrin, Salt], Wheat Gluten, Spice, Cornstarch. Fried in Vegetable Oil [Soybean, Cottonseed, Corn, and/or Canola Oil]), Soybean Oil (Hydrogenated Soybean Oil Chicken Egg Roll with TBHQ and Citric Acid added as preservatives, and Dimethylpolysiloxane added as an anti-foaming agent). Contains Egg, Soy, Wheat. Vegetable Egg Roll (Cabbage, Enriched Flour [{Bleached Wheat Flour, Niacin, Reduced Iron, Thiamine Mononitrate, Riboflavin, Folic Acid}, Calcium Propionate, Malted Barley Flour], Water, Carrots, Broccoli, Water Chestnuts, Enriched Durum Flour [Wheat Flour, Niacin, Ferrous Sulfate, Thiamine Mononitrate, Riboflavin, Folic Acid], Brown Rice, Celery, Bamboo Shoots, Contains 2% or Less of: Salt, Modified Food Starch, Onion, Yeast Extract [Yeast Extract, Sunflower Oil], Toasted Sesame Oil, Flavoring [Yeast Extract, Salt, Maltodextrin, Natural and Artificial Flavor], Vegetable Oil [Cottonseed and/or Canola Oil], Dried Garlic, Wheat Gluten, Sugar, Spice, Eggs, Cornstarch. Fried in Vegetable Oil (Cottonseed and/or Canola Oil), Soybean Oil (Hydrogenated Veggie Egg Roll Soybean Oil with TBHQ and Citric Acid added as preservatives, and Dimethylpolysiloxane added as an anti-foaming agent). Contains Egg, Wheat. -

Evaluation of Soft Spreadable Margarine Properties Produced by Lipase-Catalysed Interesterification of Chicken Fat and Corn Oil
International Food Research Journal 19 (3): 801-806 (2012) Evaluation of soft spreadable margarine properties produced by lipase-catalysed interesterification of chicken fat and corn oil 1*Afida, T. and2 Mamot, S. 1Department of Chemistry , Jalan Sultan 46661, Petaling Jaya, Selangor 2Food Science Programme, School of Chemical Sciences and Food Technology, Faculty of Science and Technology, University Kebangsaan Malaysia, Bangi, Selangor Malaysia Abstract: Chicken fat is a potential bioresource that can be developed into a commercial product. In this study, chicken fat, which is rich in unsaturated fatty acids, including oleic acid (C18:1) and linoleic acid (C18:2), was enzymatically interesterified with corn oil to produce a soft spread. Two interesterified products, sample 16 (4% enzyme, 4:1 mole ratio of chicken fat to corn oil, 50°C and 42 h of the interesterification process) and sample 17 (4% enzyme, 2:1 mole ratio of chicken fat to corn oil, 30°C and 42 h of the interesterification process), were selected based on the highest SFC at 30oC which were close to SFC values of commercial product. A morphological study showed that the final products had smaller and less dense fat particles, which explained the lower melting temperatures and solid fat content (3.2 and 3.5% for samples 16 and 17, respectively, at 20°C) compared to the commercial products (9.7, 6.8 and 7.7% for products A, B and C, respectively, at 20°C). However, both sample 16 and 17 had similar thermal properties to a vegetable-oil-based commercial product, with melting enthalpies (ΔH) of 58.45 J/g and 71.40 J/g, and were fully melted at 31.40°C and 35.41°C, respectively. -

Fat and Fatty Acid Content of Selected Foods Containing Trans-Fatty Acids
Fat and Fatty Acid Content of Selected Foods Containing Trans-Fatty Acids Special Purpose Table No. 1 NOTE: The samples analyzed for this table were collected between 1989 and 1993. As the formulations for these products may have changed, caution should be exercised when using these values. Prepared by: Jacob Exler, Linda Lemar, and Julie Smith U.S. Department of Agriculture Agricultural Research Service Beltsville Human Nutrition Research Center Nutrient Data Laboratory 10300 Baltimore Ave. B-005, Rm. 107, BARC-West Beltsville, MD 20705 Tel. 301-504-0630 FAX: 301-504-0632 This table provides analytical data on selected foods containing trans-fatty acids. All of the data presented were obtained under USDA contract. The samples were analyzed by capillary gas-liquid chromatography, and the studies were monitored for quality control. More detailed information on the analytical methodology and quality control can be obtained from the authors at the above address. Codes indicating the source of the data, descriptions of food items, and listings of the fat added as declared on the food labels are given in file below. Codes for sources of data are given for each item. Generic descriptions are provided for all food items reported. The items are arranged by major food category that correspond to the sections of Agriculture Handbook No. 8. A list of the categories is given below. For a given food description, all items bearing the same alphabetic brand designation are the same brand name item. The letter "h" in the fat added field indicates that the fat was either hydrogenated or partially hydrogenated. -

Differentiation of Lard from Other Edible Fats by Gas Chromatography- Flame Ionisation Detector (GC-FID) and Chemometrics
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Journal of Food and Pharmaceutical Sciences J.Food Pharm.Sci. 2 (2014): 27-31 Avalaible online at www. jfoodpharmsci.com Research Article Differentiation of Lard from Other Edible Fats by Gas Chromatography- Flame Ionisation Detector (GC-FID) and Chemometrics Omar Dahimi1, Mohd. Sukri Hassan1, Alina Abdul Rahim1*, Sabo Mohammed Abdulkarim2, and Siti Mashitoh A.1 1Institute of Halal Research and Management (IHRAM), Universiti Sains Islam Malaysia, 71800, Nilai, Negeri Sembilan, Malaysia 2Faculty of Food Science and Technology, Universiti Putra Malaysia, 43400 Serdang, Selangor, Malaysia. ARTICLE INFO ABSTRACT The presence of lard or its derivatives in any food products is a serious Received 05 December 2012 religious issue among Muslim and Judaism. Thus, the objective of this study Revised form 27 December 2012 was to investigate the use of gas chromatography with flame ionisation Accepted 02 January 2013 detector (GC-FID) coupled with chemometrics techniques such as Principle Available online 12 May 2013 Components Analysis (PCA) and K-mean cluster analysis to differentiate lard adulteration at very low concentrations in beef and chicken fats. The measurements were made from the pure lard, beef tallow, pure chicken fat; and beef tallow (BT), chicken fat (CF) adulterated with different concentrations of lard (0.5%-10% in BT and CF). The data were first scaled into standardisation before PCA is performed to each of the scaled data using Unscrambler software. The Scores plots and loadings plots of each scaled data were compared and studied. The results showed that lard contains higher fatty acid (FA) of C18: 2cis and low C16:0 FA, but oppositely for beef tallow and chicken fat. -

Ingredient Book 11-4-2020.Pdf
The following is a list of ingredients used in various recipes that are served to clients of Meals on Wheels as dictated through our menus. Regular and modified menus are approved by a registered dietitian to ensure proper nutrition for older adults. It is the client’s responsibility to ensure physician’s recommendations are followed. Every effort will be made to provide the selected menu, but occasionally there may be a substitution served due to circumstances beyond our control. Menus are subject to change. In addition, substitute ingredients may be used due to circumstances beyond our control with vendors. Exact serving sizes and detailed nutrition information is available upon request. For questions or concerns about the menu or for the most up to date ingredients please call Amber Goines at 740-681-5050. 11/4/2020 Beef BEEF, STRIP FAJITA SEASONED COOKED FROZEN SMARTSERVE INGREDIENTS BEEF, WATER, SEASONING [SALT, MALTODEXTRIN, SODIUM PHOSPHATE, GARLIC, SPICES, DISODIUM INOSINATE AND DISODIUM GUANYLATE, PAPAIN], VEGETABLE PROTEIN PRODUCT [SOY PROTEIN CONCENTRATE, ZINC OXIDE, NIACINAMIDE, FERROUS SULFATE, COPPER GLUCONATE, VITAMIN A PALMITATE, CALCIUM PANTOTHENATE, THIAMINE MONONITRATE B1, PYRIDOXINE HYDROCHLORIDE B6, RIBOFLAVIN B2, CYANOCOBALAMIN B12], CONTAINS 2 OR LESS OF THE FOLLOWING CANOLA OIL WITH TBHQ AND CITRIC ACID, DIMETHYLPOLYSILOXANE], BLACK PEPPER, DEXTROSE, SODIUM PHOSPHATE, YEAST EXTRACT, NATURAL FLAVOR. CONTAINS SOY BEEF, TOP INSIDE ROUND PACKER INGREDIENTS NOT AVAILABLE AT THIS TIME BEEF, PATTY GROUND STEAK 78/22 4:1 HOMESTYLE RAW FROZEN CLOUD THE CLOUD BEEF BEEF, BREADED STEAK COUNTRY FRIED LOW SALT COOKED FROZEN ADVANCE FOOD HEARTLAND BEEF BEEF, SALT, POTASSIUM AND SODIUM PHOSPHATE. BREADED WITH: ENRICHED BLEACHED WHEAT FLOUR ENRICHED WITH NIACIN, FERROUS SULFATE, THIAMINE MONONITRATE, RIBOFLAVIN, FOLIC ACID, SPICES, SALT, LEAVENING SODIUM ALUMINUM PHOSPHATE, SODIUM BICARBONATE, TORULA YEAST, SOYBEAN OIL, ONION POWDER. -

What Are “Oils”?
What are “oils”? Oils are fats that are liquid at room temperature, like the vegetable oils used in cooking. Oils come from many different plants and from fish. Some common oils are: • canola oil • corn oil • cottonseed oil • olive oil • safflower oil • soybean oil • sunflower oil Some oils are used mainly as flavorings, such as walnut oil and sesame oil. A number of foods are naturally high in oils, like: • nuts • olives • some fish • avocados Foods that are mainly oil include mayonnaise, certain salad dressings and soft (tub or squeeze) margarine with no trans fats. Check the Nutrition Facts label to find margarines with 0 grams of trans fat. Most oils are high in monounsaturated or polyunsaturated fats, and low in saturated fats. Oils from plant sources (vegetable and nut oils) do not contain any cholesterol. In fact, no foods from plants sources contain cholesterol. A few plant oils, however, including coconut oil and palm kernel oil, are high in saturated fats and for nutritional purposes should be considered to be solid fats. Solid fats are fats that are solid at room temperature, like butter and shortening. Solid fats come from many animal foods and can be made from vegetable oils through a process called hydrogenation. Some common solid fats are: • butter • beef fat (tallow, suet) • chicken fat • pork fat (lard) • stick margarine • shortening How are oils different from solid fats? All fats and oils are a mixture of saturated fatty acids and unsaturated fatty acids. Solid fats contain more saturated fats and/or trans fats than oils. Oils contain more monounsaturated (MUFA) and polyunsaturated (PUFA) fats. -

RENEWABLE DIESEL Tallow Filtration Applications
RENEWABLE DIESEL Tallow Filtration Applications Renewable diesel is an advanced biofuel About one-third of the fats and oils produced in the United States are animal fats including beef tallow, pork lard, that is biodegradable. It is a cleaner-burning, and chicken fat. Animal fats are attractive feedstocks for renewable diesel because their cost is substantially lower drop-in replacement to petroleum diesel fuel than the cost of vegetable oil and animal fat feedstocks can and can be used in vehicles and heating be made into high-quality diesel. systems. Renewable diesel is primarily Because it has the same chemical structure as petroleum diesel, renewable diesel can be used in engines that are produced from animal fats, inedible corn oil, designed to run on conventional diesel fuel —with no blending required. and vegetable oils. Waste fat from animal carcasses are removed and then made into an oil using a rendering process. Rendering consists of grinding the animal by-products to a fine consistency and The Benefits of an optimized filtration cooking them until the liquid fat separates and pathogens are system include: destroyed. • Improve operation efficiency by delivering cleanest Solid bone meal contaminants, phospholipids, or gums and possible feedstock to your hydrotreater other contaminants need to be removed to provide clean • Protect downstream equipment feedstock for the hydrotreater. This is a highly temperature • Lower operating and maintenance costs and extend life sensitive process that can make it challenging. of catalyst • Improved heat transfer and process controls • Ability to meet final product sales specification Truck Unloading Tallow Filtration 3 L. P. -

Cooking to Thrive
COOKINGOILS TO THRIVE Oils are NOT a food group but they provide essential nutrients, such as fatty acids and Vitamin E. Therefore, oils are included in recommendations for what to eat; however, only small amounts of oils are recommended. About oils: ▶ Oils are fats that are liquid at room temperature. ▶ Some commonly eaten oils include the following: canola oil, corn oil, cottonseed oil, olive oil, safflower oil, soybean oil, sunflower oil, walnut oil, and sesame oil. ▶ Some foods are naturally high in oils, like nuts, olives, some fish, and avocados. ▶ Most oils are high in monounsaturated fats (MUFA) and polyunsaturated fats (PUFA). Most of the fats you eat should be MUFAs or PUFAs. ▶ The MUFAs and PUFAs found in fish, nuts, and vegetable oils do not raise LDL (bad) cholesterol levels in the blood. How are OILS different from SOLID FATS? ▶ Solid fats are fats that are solid at room temperature, like butter and shortening. ▶ Solid fats can come from animal foods and can be made from vegetable oils through a process called hydrogenation. ▶ Examples of solid fats include the following: butter, milk fat, beef fat (tallow, suet), chicken fat, pork fat (lard), stick margarine, shortening, and partially hydrogenated oil. ▶ Solid fats contain more saturated fats and/or trans fats than oils. Saturated fats and trans fats tend to raise LDL cholesterol levels in the blood, which can increase the risk for heart disease. Tips: ▶ Eat fewer foods that are high in solid fats to help lower the risk for heart disease. ▶ Switch from solid fats to oils when preparing food. -

EQUIVALENTS, MEASUREMENTS and YIELDS Compiled by Nola Johnson Cockerham
EQUIVALENTS, MEASUREMENTS and YIELDS Compiled by Nola Johnson Cockerham NOTE: "=" means equals "-" means minus "+" means plus tsp. = teaspoon T. = tablespoon C. = cup oz. = ounce soda = baking soda MEASUREMENTS: pinch = 1/16 tsp. dash = 6 drops or 1/8 tsp. 1/4 T. = 3/4 tsp. 3 tsp. = 1 T. 1/8 C. = 2 T. = 1 fl. oz. 1/4 C. = 4 T. = 2 fl. oz. 1/3 C. = 5 T. + 1 tsp. 1/2 C. = 8 T. = 4 fl. oz. 3/4 C. = 12 T. = 6 fl. oz. 1 C. = 16 T. = 1/2 pint = 8 fl. oz. 2 C. = 1 pint = 16 fl. oz. 4 C. = 2 pints = 1 quart = 32 oz. 16 C. = 4 quarts = 1 gallon 8 quarts = 1 peck 4 pecks = 1 bushel 1 liter = 1 quart plus 3 oz. 2 oz. = 1/8 pound 4 oz. = 1/4 pound 8 oz. = 1/2 pound 16 oz. - 1 pound CAN SIZE AMOUNT USES #1/4 1/2 C. Meat spreads #1/2 1 C. Salmon #300 1 3/4 C. (14-16 oz.) #1 tall (303) 2 C. (16-17 oz.) Salmon and fruit cocktail #2 2 1/2 C. (20 oz.) Vegetables, fruits and juices #2 1/2 3 1/2 C. (29 oz.) Fruits, tomatoes, beets, pumpkin #5 6 C. (46 fluid oz.) Juices #10 12-13 C. Institutional and preparedness 1 At times it is necessary to use a substitution for a recipe ingredient. The substituted food may not perform exactly as the original food. Each ingredient has specific functions in a recipe and a substitute may alter the flavor, color, texture or volume, but still result in an acceptable finished product. -

Schmaltz by Dan Gill, Ethno-Gastronomist
Schmaltz By Dan Gill, Ethno-Gastronomist The word schmaltz is of High German origin and simply means [rendered] animal fat. Technically, lard and beef tallow are schmaltz, but the word was adopted by early Ashkenazi or European Jews and applied to poultry fats in general and specifically to chicken, duck or goose fat. Since lard is forbidden and butter restricted, schmaltz became the cornerstone of traditional Jewish cuisine and an essential ingredient in many authentic dishes, such as matzo balls and potato pancakes. Prior to the widespread use of vegetable oils and fats during the twentieth century, schmaltz was used in place of butter and lard for frying and as a spread for bread. It adds a depth and richness to most dishes that cannot be obtained with vegetable fats. In fact, there are few authentic Ashkenazi recipes that don’t call for schmaltz. In America, schmaltz also means “overly sentimental” or “mushy”. Chicken fat was held in such high regard by Jewish immigrants that merely the mention of schmaltz evokes an emotional response and fond childhood memories. Jewish comedians and performers popularized the term during the early days of radio and television. When compared to beef tallow or lard, chicken and turkey schmaltz is lower in saturated fats and higher in beneficial monounsaturated and polyunsaturated fats and essential fatty acids and is therefore healthier. Several years ago, I researched frying oils and came to the conclusion that, when heated and especially when used for frying, animal fats are actually safer and less detrimental to our health than vegetable fats (in general) because they are more stable and don’t break down into dangerous components as readily. -
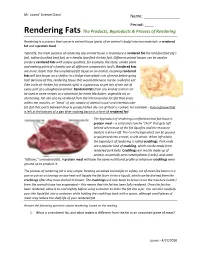
Rendering Fats the Products, Byproducts & Process of Rendering
Mr. Joanis’ Science Class! Name: ________________ Period: ____ Rendering Fats The Products, Byproducts & Process of Rendering Rendering is a process that converts animal tissue (parts of an animal’s body) into two materials: a rendered fat and a protein meal. Typically, the main purpose of rendering any animal tissue is to produce a rendered fat like lard (purified pig’s fat), tallow (purified beef fat), or schmaltz (purified chicken fat). Different animal tissues can be used to produce rendered fats with unique qualities; for example, the taste, smoke point and melting point of schmaltz are all different compared to lard’s. Rendered fats are more stable than the unrendered fat tissue on an animal, meaning rendered fats will last longer on a shelf or in a fridge than whole cuts of meat before going bad. Because of this, rendering tissue that would otherwise not be cooked to eat (like a pile of chicken fat, pictured right) is a good way to get lots of use out of every part of a slaughtered animal. Rendered fats from any kind of animal can be used in some recipes as a substitute for items like butter, vegetable oil, or shortening. Fat can also be rendered from the intramuscular fat (fat that exists within the muscles, or “meat” of any sample of animal tissue) and intermuscular fat (fat that exists between muscle groups) when any cut of meat is cooked. For example – bacon grease that is left at the bottom of a pan after cooking bacon is a form of rendered fat! The byproduct of rendering unrefined animal fat tissue is protein meal – a solid and crunchy “shell” that gets left behind when most of the fat liquefies and the moisture (water) is driven off. -

Fat Type and Amount: Its Impact on Palatability
FAT TYPE AND AMOUNT: ITS IMPACT ON PALATABILITY RESEARCH REVEALS PREFERENCE DIFFERENCES IN DOGS AND CATS FOR KIBBLE FAT SOURCES AND CONCENTRATIONS RESEARCH AND DEVELOPMENT, AFB INTERNATIONAL Overview: Dog and Cat Panel Trial Results: Fat source and Fat plays an important role in dog and cat food, providing applied percentage in order of preference nutritional, functional and palatability benefits. The type and amount of fat used varies by the diet, the finished product Preference Dog Trials: Cat Trials: specifications and the manufacturer. There has been limited Fat Source / Fat Fat Source / Fat Ranking Concentration Concentration research evaluating the correlation between palatability and E CD topical fat sources and their applied amounts, leaving many 1 Bacon / 9% Bacon / 3% pet food manufacturers questioning the potential impact 2 Beef Tallow / 9%D Chicken / 3%D various combinations may have on dog and cat preference. 3 Herring / 9%C Canola / 9%BCD To evaluate, AFB International conducted research at 4 Chicken / 9%C Herring / 3%BCD the company’s Palatability Assessment Resource Center 5 Bacon / 3%C Canola / 3%BCD (PARC) facility on the effect both external fat sources and levels have on palatability preference in dogs and 6 Beef Tallow / 3%B Beef Tallow / 9%BCD cats. The research found differences in fat sources 7 Canola / 3%AB Chicken / 9%BCD and levels preferred by dogs and cats, demonstrating AB BC that diets can be tailored based on animal preferences. 8 Chicken / 3% Beef Tallow / 3% 9 Herring / 3%AB Bacon / 9%B These insights help pet food manufacturers determine potential fat source and concentration adjustments to 10 Canola / 9%A Herring / 9%A increase palatability of their dog and cat food brands.