Risk Maps of Lassa Fever in West Africa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sierra Leone
Sierra Leone Main objectives • In collaboration with the Government of Sierra Leone and other partners, pursue the reinte- gration of Sierra Leonean returnees, leading to • Provide international protection and basic a complete phase-out of interventions by humanitarian assistance to Liberian refugees. UNHCR (i.e. rebuild national protection struc- • Facilitate the repatriation of Liberian refugees tures and hand over assistance activities to who opt to return home in conditions of safety development actors). and dignity; provide information about security and living conditions in Liberia. Planning figures • Facilitate local integration, naturalization or Population Jan 2005 Dec 2005 resettlement for Liberian refugees who arrived in Sierra Leone during the 1990s and are not Liberia (refugees) 50,000 24,000 willing to repatriate. Sierra Leonean 30,000 0 • Enhance Government capacity to handle refugee returnees issues following the adoption and implementa- Total 80,000 24,000 tion of national refugee legislation, including assisting new government structures to become Total requirements: 25,043,136 operational. UNHCR Global Appeal 2005 174 the 4Rs strategy, has yielded positive results, with Working environment the presence of the UNDP/TST (Transitional Sup- port Team) being accommodated in UNHCR field Major developments offices to ensure continuity of interventions. With the focus of reintegration efforts on consolidating In 2004, political stability and the progressive res- and linking of work already undertaken to the toration of state authority permitted a further longer-term programmes of development actors, 30,000 Sierra Leoneans to return. By 31 July 2004 UNHCR will only fund new projects in 2005 if they – the end of the organized operation launched in are sure to reach completion by the year’s end. -

MCSP Guinea Restoration of Health Services, March 2017
MCSP Guinea Restoration of Health Services, March 2017 MCSP/Rachel Waxman Guinea–Selected Demographic and Health Indicators Indicator Data Indicator Data Indicator Data TFR (births per Population (1) 10,628,972 5.1 Pneumonia (2) 38% woman) (2) CPR (modern 36.5% / Live births/year (3) 368,558 5% ORS / Zinc (2) methods) (2) 0.5% Ebola infections MMR (per 100,000 724 ANC +4 (2) 56.6% during recent 3,804 live births) (2) epidemic (4) Ebola death rate NMR (per 1,000 live (percentage of 34 SBA (2) 45.3% 66% births) (2) infections ending in death)(4) U5MR (per 1,000 live 94 DTP3 (3) 51% births) (2) Sources: (1) 2014 National Census; (2) DHS 2012; (3) WHO UNICEF 2015 coverage estimate; (4) WHO Ebola Situation Report, December 2015. Strategic Objectives: Restoration of Health Services Program At least 80% of MCSP-supported hospitals and health centers (n=221) meet minimum IPC standards. 34 facilities supported to reinvigorate and maintain the SBM-R process, with at least 80% achieving and/or maintaining recognition of performance. 75% of target facilities in the 20 focus prefectures have increased capacity to offer RMNCH services (of 26 hospitals and 195 health centers). 96 health posts supported to improve IMNCI and contribute to the care of sick children in the catchment areas of 30 health centers (revised target). 700 community health workers (ASC) are supported to reinvigorate community health and mobilization to provide health information, accompaniment of pregnant women, and FP services, as well as key lifesaving interventions; and to increase community ownership and capacity to explore, plan, and act together for improved RMNCH outcomes in communities surrounding 20 MCSP-supported health centers. -

AEMIP Quarterly Progress Report OCTOBER-DECEMBER 2014
Agriculture Education and Market Improvement Program (AEMIP) AEMIP Quarterly Progress Report OCTOBER-DECEMBER 2014 Submitted by: AEMIP Winrock International ISAV/F Campus Faranah, Guinea January 31st, 2015 AEMIP is funded by USAID Cooperative Agreement No. AID-675-A-13-00003, as an Associate Award under the Farmer-to-Farmer LWA Cooperative Agreement No.EDH-00-0900003-00. Contact AEMIP Andrew Kovarik, Program Director ISAV/F Campus Faranah, Guinea +224-62-082-0 433 [email protected] Winrock International AEMIP Quarterly Progress Report (October-December 2014) TABLE OF CONTENTS ACRONYMS ..................................................................................................................................iii I. EXECUTIVE SUMMARY–QUARTER OVERVIEW ........................................................... 2 II. WORK PLAN PROGRESS –STATUS OF PLANNED ACTIVITIES ................................ 5 A. Status of Planned Activities Year 2 ...................................................................................................................... 5 B. Discussion of planned activities that are delayed, postponed or cancelled, or unplanned activities that took place ........................................................................................................................................................ 13 III. ACCOMPLISHMENTS ....................................................................................................... 16 A. Activities implemented ......................................................................................................................................... -

G U I N E a Liberia Sierra Leone
The boundaries and names shown and the designations Mamou used on this map do not imply official endorsement or er acceptance by the United Nations. Nig K o L le n o G UINEA t l e a SIERRA Kindia LEONEFaranah Médina Dula Falaba Tabili ba o s a g Dubréka K n ie c o r M Musaia Gberia a c S Fotombu Coyah Bafodia t a e r G Kabala Banian Konta Fandié Kamakwie Koinadugu Bendugu Forécariah li Kukuna Kamalu Fadugu Se Bagbe r Madina e Bambaya g Jct. i ies NORTHERN N arc Sc Kurubonla e Karina tl it Mateboi Alikalia L Yombiro Kambia M Pendembu Bumbuna Batkanu a Bendugu b Rokupr o l e Binkolo M Mange Gbinti e Kortimaw Is. Kayima l Mambolo Makeni i Bendou Bodou Port Loko Magburaka Tefeya Yomadu Lunsar Koidu-Sefadu li Masingbi Koundou e a Lungi Pepel S n Int'l Airport or a Matotoka Yengema R el p ok m Freetown a Njaiama Ferry Masiaka Mile 91 P Njaiama- Wellington a Yele Sewafe Tongo Gandorhun o Hastings Yonibana Tungie M Koindu WESTERN Songo Bradford EAS T E R N AREA Waterloo Mongeri York Rotifunk Falla Bomi Kailahun Buedu a i Panguma Moyamba a Taiama Manowa Giehun Bauya T Boajibu Njala Dambara Pendembu Yawri Bendu Banana Is. Bay Mano Lago Bo Segbwema Daru Shenge Sembehun SOUTHE R N Gerihun Plantain Is. Sieromco Mokanje Kenema Tikonko Bumpe a Blama Gbangbatok Sew Tokpombu ro Kpetewoma o Sh Koribundu M erb Nitti ro River a o i Turtle Is. o M h Sumbuya a Sherbro I. -

Post-Ebola Community Health Worker Programme Performance In
F1000Research 2019, 8:794 Last updated: 28 SEP 2021 RESEARCH ARTICLE Post-Ebola Community Health Worker programme performance in Kenema District, Sierra Leone: A long way to go! [version 1; peer review: 1 approved, 1 approved with reservations] Harold Thomas1, Katrina Hann 2, Mohamed Vandi1, Joseph Bengalie Sesay3, Koi Sylvester Alpha4, Robinah Najjemba 5 1Directorate of Health Security and Emergencies, Ministry of Health and Sanitation, Freetown, Sierra Leone 2Sustainable Health Systems, Freetown, Sierra Leone 3Koinadugu District Health Management Team, Ministry of Health and Sanitation, Kabala, Sierra Leone 4Kenema District Health Management Team, Ministry of Health and Sanitation, Kenema, Sierra Leone 5Makerere University School of Public Health, Makerere, Uganda v1 First published: 06 Jun 2019, 8:794 Open Peer Review https://doi.org/10.12688/f1000research.18677.1 Latest published: 09 Apr 2020, 8:794 https://doi.org/10.12688/f1000research.18677.2 Reviewer Status Invited Reviewers Abstract Background: The devastating 2014-2015 Ebola outbreak in Sierra 1 2 Leone could erode the gains of the health system including the Community Health Worker (CHW) programme. We conducted a study version 2 to ascertain if the positive trend in reporting cases of malaria, (revision) report pneumonia and diarrhoea treated by CHWs in the post-Ebola period 09 Apr 2020 has been sustained 18 months post-Ebola. Methods: We conducted a retrospective cross-sectional study using version 1 aggregated CHW programme data (2013-2017) from all Primary 06 Jun 2019 report report Health Units in Kenema district. Data was extracted from the District Health Information System and analysed using STATA. Data in the pre- (June 2013-April 2014), during- (June 2014-April 2015) and post-Ebola 1. -

A Methodology for Complex Agricultural Development Projects
Systemic Impact Evaluation: A Methodology for Complex Agricultural Development Projects. The Case of a Contract Farming Project in Guinea Jocelyne Delarue, Hubert Cochet To cite this version: Jocelyne Delarue, Hubert Cochet. Systemic Impact Evaluation: A Methodology for Complex Agri- cultural Development Projects. The Case of a Contract Farming Project in Guinea. The European Journal of Development Research, 2013, 25 (5), p.778-796. 10.1057/ejdr.2013.15. halshs-01374496 HAL Id: halshs-01374496 https://halshs.archives-ouvertes.fr/halshs-01374496 Submitted on 7 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Original Article Systemic Impact Evaluation: A Methodology for Complex Agricultural Development Projects. The Case of a Contract Farming Project in Guinea Jocelyne Delaruea and Hubert Cochetb aAgence Française de Développement, Evaluation and Capitalization Unit, Paris, France. E-mail: [email protected] bAgro Paris Tech, Comparative Agriculture and Agricultural Development Research Unit, Paris, France. E-mail: [email protected] Abstract This article presents a mixed-method approach used to analyse the impact of a complex agricultural development project, the SOGUIPAH (Guinean Oil Palms and Rubber Company) project designed to promote oil palm and rubber cultivation in Guinea. -
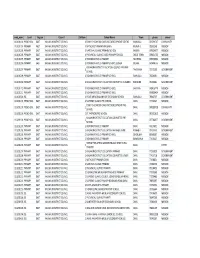
2016 School List.Xlsx
emis_num Level Region Council Chfdom School Name Town phone owner 110101101 PRESCHOOL EAST KAILAHUN DISTRICT COUNCIL 1 EARLY CHILDHOOD CARE AND DEVELOPMENT CENTRE BAIWALLA 076593767 COMMUNITY 110101201 PRIMARY EAST KAILAHUN DISTRICT COUNCIL 1 METHODIST PRIMARY BAIWALA BAIWALA 78963548 MISSION 110101202 PRIMARY EAST KAILAHUN DISTRICT COUNCIL 1 NATIONAL ISLAMIC PRIMARY SCHOOL BAOMA 078624877 MISSION 110101203 PRIMARY EAST KAILAHUN DISTRICT COUNCIL 1 PROVINCIAL ISLAMIC DODO PRIMARY SCHOOL DODO TOWN 078451705 MISSION 110101205 PRIMARY EAST KAILAHUN DISTRICT COUNCIL 1 ROMAN CATHOLIC PRIMARY NAGBENA 078360004 MISSION 110101206 PRIMARY EAST KAILAHUN DISTRICT COUNCIL 1 ROMAN CATHOLIC PRIMARY SCHOOL SIENGA SIENGA 076484775 MISSION KAILAHUN DISTRICT EDUCATION COUNCIL PRIMARY 110101207 PRIMARY EAST KAILAHUN DISTRICT COUNCIL 1 TAKPOIMA 79175290 GOVERNMENT SCHOOL 110101208 PRIMARY EAST KAILAHUN DISTRICT COUNCIL 1 ROMAN CATHOLIC PRIMARY SCHOOL BAIWALLA 76606361 MISSION 110101209 PRIMARY EAST KAILAHUN DISTRICT COUNCIL 1 KAILAHUN DISTRICT EDUCATION COMMITTEE KURANKO KURANKO 76735861 GOVERNMENT 110101210 PRIMARY EAST KAILAHUN DISTRICT COUNCIL 1 ROMAN CATHOLIC PRIMARY SCHOOL SAKIEMA 078456779 MISSION 110101211 PRIMARY EAST KAILAHUN DISTRICT COUNCIL 1 ROMAN CATHOLIC PRIMARY SCHOOL 076820424 MISSION 110101301 JSS EAST KAILAHUN DISTRICT COUNCIL 1 PEACE MEMORIAL JUNIOR SECONDARY SCHOOL BAIWALLA 78540707 GOVERNMENT 110201101 PRESCHOOL EAST KAILAHUN DISTRICT COUNCIL 2 SUPREME ISLAMIC PRE‐SCHOOL DARU 77702647 MISSION EARLY CHILDHOOD CARE AND DEVELOPMENT PRE‐ 110201102 -
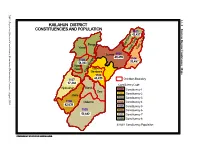
Kailahun District Constituencies And
NEC: Report on Electoral Constituency Boundaries Delimitation Process Process Delimitation Boundaries Constituency Electoral on Report NEC: 4.1.1 KAILAHUN DISTRICT CONSTITUENCIES AND POPULATION Eastern Region Constituency Maps 1103 a 43,427 m i a g g n K n e i o s T s T i i i s Penguia s K is is Yawei K K Luawa 1101 e 49,499 r 1104 1108 g n 33,457 54,363 o B Kpeje je e Upper West p K Bambara 1102 44,439 1107 Chiefdom Boundary 37,484 Constituency Code Njaluahun Mandu – 1101 Constituency 1 August 2006 August Dea 1102 Constituency 2 Jawie 1103 Constituency 3 1106 Malema 1104 Constituency 4 42,639 1105 Constituency 5 1105 1106 Constituency 6 52,882 1107 Constituency 7 1108 Constituency 8 42,639 Constituency Population PREPARED BY STATISTICS SIERRA LEONE KENEMA DISTRICT CONSTITUENCIES AND POPULATION Gorama Mende 1207 49,953 Wandor 1206 48,429 n u h Simbaru o g Lower le 1208 Dodo Bambara a M 54,312 1205 42,184 Kandu Leppiama 1204 51,486 1202 1201 42,262 Nongowa 43,308 # Small Bo # Kenema # 1203 1209 Town 42,832 44,045 Dama 1210 Niawa 36341 Gaura Langrama Koya 1211 Nomo 42,796 Chiefdom Boundary Constituency Code Tunkia 1201 Constituency 1 1202 Constituency 2 1203 Constituency 3 1204 Constituency 4 1205 Constituency 5 1206 Constituency 6 1207 Constituency 7 1208 Constituency 8 1209 Constituency 9 1210 Constituency 10 1211 Constituency 11 42,796 Constituency Population PREPARED BY STATISTICS SIERRA LEONE NEC: Report on Electoral Constituency Boundaries Delimitation Process – August 2006 NEC: Report on Electoral Constituency Boundaries Delimitation -

U N I T E D N a T I O
U N I T E D N A T I O N S Office for the Coordination of Humanitarian Affairs SIERRA LEONE HUMANITARIAN SITUATION REPORT SEPTEMBER 2003 KEY EVENTS district. A concern raised in Kono is that none of the Watsan implementing partners had the facilities or machines for testing water • Yellow Fever outbreak samples. This has been reported to the MOHS. • Security Council extends UNAMSIL’s mandate • UN Agencies and GoSL celebrate World Peace Day SECURITY HIGHLIGHTS • Nigerian lawmakers call on UNAMSIL Overall security UNAMSIL (United Nations Mission in Sierra Leone) reports the overall security situation in HUMANITARIAN HIGHLIGHTS the country to be calm. However there have been some concerns about security along the Yellow Fever outbreak border regions, particularly along the Mano The Ministry of Health and Sanitation (MOHS) River Union Bridge in the south. Similarly the has reported a total of 90 cases of Yellow Sierra Leone Police (SLP) are concerned Fever, from eight districts in the country: about the porous nature of the border in the Tonkolili, Bombali, Kenema, Koinadugu, Port Kamakwie, Tambakha and Koinadugu areas, Loko, Kambia and Kono. Of the 90 reported in the Northern Province that have resulted in cases (as of 29 September) four laboratory increased smuggling of goods across the cases were confirmed, all from the Tonkolili borders. The police have also reported hunters District, where majority of the suspected cases from Guinea, coming across, poaching and emanate from. Earlier, the MOHS gave out crossing back into Guinea. 100,000 doses of vaccine in four chiefdoms in the district. They have now finally secured UNAMSIL’s mandate extended funds to carry out mass immunization The UN Security Council has extended campaign in the remaining seven chiefdoms of UNAMSIL’s mandate, which was to expire on the district. -

The Heart of the Matter
THE HEART OF THE MATTER SIERRA LEONE, DIAMONDS & HUMAN SECURITY (COMPLETE REPORT) Ian Smillie Lansana Gberie Ralph Hazleton Partnership Africa Canada (PAC) is a coalition of Canadian and African organizations that work in partnership to promote sustainable human development policies that benefit African and Canadian societies. The Insights series seeks to deepen understanding of current issues affecting African development. The series is edited by Bernard Taylor. The Heart of the Matter: Sierra Leone, Diamonds and Human Security (Complete Report) Ian Smillie, Lansana Gberie, Ralph Hazleton ISBN 0-9686270-4-8 © Partnership Africa Canada, January 2000 Partnership Africa Canada 323 Chapel St., Ottawa, Ontario, Canada K1N 7Z2 [email protected] P.O. Box 60233, Addis Ababa, Ethiopia pac@ telecom.net.et ________________ The Authors Ian Smillie, an Ottawa-based consultant, has 30 years of international development experience, as manager, programmer, evaluator and writer. He was a founder of the Canadian NGO Inter Pares, and was Executive Director of CUSO from 1979 to 1983. His most recent publications include The Alms Bazaar: Altruism Under Fire; Non Profit Organizations and International Development (IT Publications, London, 1995) and Stakeholders: Government-NGO Partnerships for International Development (ed. With Henny Helmich, Earthscan, London, 1999). Since 1997 he has worked as an associate with the Thomas J. Watson Jr. Institute at Brown University on issues relating to humanitarianism and war. Ian Smillie started his international work in 1967 as a teacher in Koidu, the centre of Sierra Leone’s diamond mining area. Lansana Gberie is a doctoral student at the University of Toronto and research associate at the Laurier Centre for Military, Strategic and Disarmament Studies, Waterloo, Ontario. -

West Africa – Ebola Outbreak Fact Sheet #23, Fiscal Year (Fy) 2015 March 4, 2015
WEST AFRICA – EBOLA OUTBREAK FACT SHEET #23, FISCAL YEAR (FY) 2015 MARCH 4, 2015 NUMBERS AT HIGHLIGHTS USG HUMANITARIAN ASSISTANCE A GLANCE TO EVD OUTBREAK RESPONSE WHO reports 132 new confirmed cases of TO DATE IN FY 2014 & FY 2015 EVD in Guinea and Sierra Leone in the 23,934 week ending on March 1. USAID/OFDA1 $463,846,949 No new EVD cases confirmed in Liberia USAID/FFP2 $57,479,546 Number of Suspected, in nearly two weeks. Probable, and Confirmed USAID/GH3 $20,076,000 Ebola Virus Disease (EVD) Response actors remain concerned Cases in Acutely Affected regarding ongoing EVD transmission USAID/Liberia $16,100,000 Countries* chains in Guinea’s Forécariah Prefecture, USAID/Guinea $3,482,000 UN World Health Organization where 23 EVD cases since February 28— (WHO) – DoD4 $353,958,000 March 4, 2015 more than 70 percent of the 32 confirmed cases in the same time period CDC5 $131,455,1156 countrywide—have occurred. 9,792 $1,046,397,610 Number of EVD-Related USG ASSISTANCE TO THE WEST AFRICA 7 Deaths* EVD OUTBREAK RESPONSE WHO – March 4, 2015 KEY DEVELOPMENTS 11,466 As of March 3, the Government of Liberia (GoL) Incident Management System (IMS) had Number of EVD Cases in not reported a confirmed case of EVD in 12 days, with the most recent case documented in Sierra Leone* Montserrado County on February 19. On February 27, the IMS announced that Liberia had WHO – March 4, 2015 entered Phase 3 of the EVD response, which will focus on restoring non-EVD health services and improving triage and referral processes to enable medical staff to safely identify and isolate suspect EVD cases. -

Sierra Leone
Sierra Leone A TAG LANCE Main Objectives and Activities Assess the protection requirements of those Liberian refugees who did not wish to repatriate; facilitate the local integration of Liberian refugees in need of international protection and identify resettle- ment opportunities; assess how national and local socio-economic, political and security factors will affect the needs of Sierra Leonean returnees; compile Returnee District Area Profiles taking into account the security needs of potential returnees to those dis- Persons of Concern tricts; facilitate and later promote Main Refugee Total Of whom Per cent Per cent Origin/Type of in UNHCR Female under 18 the voluntary repatriation of Sierra Population Country assisted Leonean refugees and help them Sierra Leone (IDPs) 300,000 - - - settle back into their communities Sierra Leone 200,000 - - - of origin; in the context of the (Returned IDPs) Brookings process, establish opera- Sierra Leone 40,900 24,900 - - tional linkages and mechanisms (Returnees) which best ensure a smooth transi- Liberia (Refugees) 6,500 6,500 - - tion from emergency relief to longer-term reconstruction and recovery. Income and Expenditure (USD) Annual Programme, Trust Funds and Supplementary Programme Budgets Impact Revised Income Other Total Total • In 2000, UNHCR undertook Budget from Funds Funds Expenditure Contributions1 Available2 Available screening of 1,872 Liberian AB/TF 941,439 879,376 431,354 1,310,730 832,471 households including new appli- SB 9,380,111 3,966,962 2,697,173 6,664,135 6,664,135 cations, in order to determine the Total 10,321,550 4,846,338 3,128,527 7,974,865 7,496,606 need of those who did not partic- 1Includes income from contributions earmarked at the country level.