Session of Codex Committee on Fats and Oils (CCFO) Held in Langkawi, Malaysia from 25 February-1 March 2013
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pistachio Oil
Pistachio Oil Oleum Pistaciae synonyms: Pistazien(kern)öl (D); huile de pistache (F) 1 Source Plant Pistacia vera L. (Anacardiaceae), pistachio © Springer Nature Switzerland AG 2020 593 S. Krist, Vegetable Fats and Oils, https://doi.org/10.1007/978-3-030-30314-3_94 594 Pistachio Oil Habitat P Pistacia vera originates in Central Asia and the Mediterranean area. Alexander the Great brought it to Greece, and the Romans brought to to Sicily. Even today, pista- chios grow wild in several countries, for example in Afghanistan and India. They prefer dry, desert-like regions and are very frost-susceptible. Pistacia vera has a biennial crop sequence, which is why there are in turn small and large amounts of crop (Hager 1978, volume 6a, p. 730; Roth and Kormann 2000, p. 145). Description Pistacia vera is an evergreen, deciduous tree that can reach a height of 8–12 m. The crown is spreading and forms a dense canopy of leaves. The leaves are pinnate or bipinnate, greyish green, with stalkless, ovate leaflets. The panicle is short; the inconspicuous, axillary flowers are a reddish colour. They develop into elongated, oval stone fruit that are about 2–3 cm long. The fruit are brownish red and wrinkly, with a thin layer of fruit pulp that tastes of turpentine. They contain the seeds that are sold as pistachios or “green almonds”. The seed is usually triangular, a green, brownish or violet colour and 20 mm long. It is slightly compressed at the sides and protected by a whitish, hard shell (Hager 1978, volume 6a, p. -

Consumer Acceptance and Quantitative Descriptive Analysis of Pistachio Spread
J. Agr. Sci. Tech. (2017) Vol. 19: 85-95 Consumer Acceptance and Quantitative Descriptive Analysis of Pistachio Spread A. Shakerardekani1 ABSTRACT Pistachio nut (Pistacia vera L.) is one of the popular and nutritious tree nuts in the world. Pistachio spread is a new product which is made from pistachio paste, icing sugar, Soy Protein Isolate (SPI), and Red Palm Oil (RPO). This study involved sensory acceptability (by 32 assessors) using Hedonic scale and development of suitable terminology for describing pistachio spread using Quantitative Descriptive Analysis (QDA). This study represents the first report on using QDA for sensory evaluation of pistachio products. The QDA method is used to determine the sensory profile of the two pistachio spreads with higher acceptability in the Hedonic scale (Formulation 12 including 50% pistachio paste, 30% icing sugar, and 20% RPO and Formulation 16 including 58.3% pistachio paste, 25% icing sugar, and 16.7% RPO). According to the results, RPO has a direct effect on the sensory acceptance of pistachio spread (P< 0.05). Eight panelists were selected for evaluation of pistachio spread. Twenty attributes (in terms of appearance (green color, visible particles, glossy), aroma (sweet, roasted, nutty, milky/creamy), flavor taste (beany, sweet, oily, bitter, nutty, creamy), texture (stickiness, oiliness, firmness, adhesiveness, spreadability), and aftertaste (bitter, astringency) were identified and developed for the product. No significant difference was observed in all pistachio spread formulations attributes, except for sweetness (P< 0.05). Keywords: Hedonic test, Panelist, QDA, Red palm oil, Sensory evaluation. INTRODUCTION The split pistachios are consumed as roasted and/or salted nut snacks. -

Specialty Oils Is Preventing That Remains After Pressing, and Virgin Olive Oxidation―Especially in Those That Contain a High Level of Oil
[Fats/Oils] Vol. 18 No. 9 September 2008 Pouring on Specialty Oil Ideas By Tamie Cook, Contributing Editor They say variety is the spice of life. And, if that’s the case, the product and menu developer’s pantry can be a veritable smorgasbord of piquancy. Literally, we have the world at the end of our whisk, and this has never been truer than when it comes to reaching for oils. In days past, choices were largely limited to vegetable, peanut, soybean, the occasional variety of olive oil and, on a good day, extra virgin olive oil. No longer confined to these basics, myriad choices now address today’s ingredient requirements for health, variety, taste and versatility. Whether it’s for a new dish on the menu or the next big product line, look at the choices, learn about their many uses and try some new oil on your palette. Most fruit, nut and seed oils generally undergo comparatively little processing. Many are mechanically, or “expeller,” extracted and are unrefined so no solvent was used to extract the oil, and no bleach or deodorizing agent was used to refine it. Thus, the oil retains its full, natural flavor, aroma and color, and many of its naturally occurring nutrients. This translates into a product that not only adds flavor to a dish, but also adds value to a menu or product. Shedding new light on an old friend When it comes to olive oil, everything old is new again. The olive tree, a native of Asia Minor, has been around for more than 6,000 years. -

Pistachio Consumption Prevents and Improves Lipid Dysmetabolism by Reducing the Lipid Metabolizing Gene Expression in Diet-Induced Obese Mice
nutrients Article Pistachio Consumption Prevents and Improves Lipid Dysmetabolism by Reducing the Lipid Metabolizing Gene Expression in Diet-Induced Obese Mice Simona Terzo 1, Gaetano Felice Caldara 1, Vincenzo Ferrantelli 2, Roberto Puleio 2 , Giovanni Cassata 2, Flavia Mulè 1 and Antonella Amato 1,* 1 Dipartimento di Scienze e Tecnologie Biologiche, Chimiche e Farmaceutiche (STEBICEF), Università di Palermo, viale delle Scienze, Edificio 16, 90128 Palermo, Italy; [email protected] (S.T.); [email protected] (G.F.C); fl[email protected] (F.M.) 2 Istituto Zooprofilattico Sperimentale della Sicilia “A. Mirri”, Via Gino Marinuzzi 3, 90129 Palermo, Italy; [email protected] (V.F.); [email protected] (R.P.); [email protected] (G.C.) * Correspondence: [email protected]; Tel.: +39-091-2389-7506 Received: 20 October 2018; Accepted: 16 November 2018; Published: 1 December 2018 Abstract: Pistachios contain beneficial substances such as unsaturated fatty acids, phytosterols, and polyphenols. In the present study, we investigated if pistachio consumption is able to prevent or to revert hyperglycemia, dyslipidemia, hepatic steatosis, and adipose tissue morphological alterations caused by high fat diet (HFD) in the mouse. Moreover, the impact of pistachio intake on the mRNA expression of peroxisome proliferator-activated receptor γ (PPAR-g), fatty acid transport proteins (FAT-P), fatty acid synthase (FAS), stearoyl-CoA desaturase (SCD1), and sterol regulatory element-binding transcription factor-1c (SREBP-1c) in liver and adipose tissue was also analyzed. No change in body weight, food intake, and hyperglycemia was observed between mice consuming pistachios (HFD-P) and HFD mice. Pistachio intake was able to prevent but not to reverse HFD-induced hypertriglyceridemia. -

Bma Usa Loc 12-5-201
סב '' ד ד ׳ לסכ ו שת פ׳׳ December 5, 2019 To Whom it may concern: This is to certify that the following 64 products: BRAND PRODUCT MASSIMO gusto Extra Virgin Olive Oil MASSIMO gusto Pure Grape Seed Oil MASSIMO gusto Blended Grape Seed Oil MASSIMO gusto Extra Virgin Avocado Oil MASSIMO gusto Extra Virgin Avocado Oil with Garlic MASSIMO gusto Blended Vegetable Oil 75% Canola Oil 25% Extra Virgin Olive Oil MASSIMO gusto Extra Virgin Avocado Oil Blend MASSIMO gusto Balsamic Vinegar of Modena MASSIMO gusto Mediterranean Blend MASSIMO gusto Pomace Oil MASSIMO gusto Pure Olive Oil MASSIMO gusto Pomace Olive Oil MASSIMO gusto Grape Seed Oil MASSIMO gusto Canola Oil MASSIMO gusto Soybean Oil MASSIMO gusto Rice Bran Oil MG Estate Reserve Organic Extra Virgin Olive Oil First Cold Pressed MG Estate Reserve Extra Virgin Avocado Oil MG Estate Reserve Grape Seed Oil MG Pure Grape Seed Oil MG Walnut Oil MG Sweet Almond Oil MG Safflower Oil MG Roasted Sesame Oil MG Flax Seed Oil MG Peanut Oil MG Rice Bran Oil MG Limited Reserve Extra Virgin Olive Oil First Cold Pressed Californian Grown MG Limited Reserve First Cold Pressed Avocado Oil with Garlic MG Organic Extra Virgin Olive Oil Infuse with Lemon MG Organic Extra Virgin Olive Oil Infuse with Lime MG Organic Extra Virgin Olive Oil Infuse with Orange North American Kosher Supervision (Flag K) BMA USA Kosher Certificate 5780 Page 1 of 3 MG Organic Extra Virgin Olive Oil Infused with Jalapeno Pepper MG Organic Extra Virgin Olive Oil Infused with Garlic MG Organic Extra Virgin Olive Oil Infused with Basil -

Pistachio Oil Cold Pressed Virgin Oil
Pistachio Oil cold pressed virgin oil INCI Name: Pistacia Vera Seed Oil Furthermore, pistachio oil is a natural source of Stigmasterol and Δ-5-Avenasterol, linoleic acid and Key Benefits: β-sitosterol, which are 5α-reductase inhibitors, as well • Anti-oxidant properties as effective lenitive compounds. Moreover, linoleic • Rich in emollient properties acid helps to re-fat and boost the intercorneocyte • Hydration lipids. • Strengthens hair An additional benefit of pistachio oil is its ability to Background help strengthen hair over time. Pistachio oil protects Native to the Middle East, pistachios are one of and repairs the hair shaft, prevents split ends, and the oldest flowering nut trees. Ancient people is an effective detangler for all hair types. Thanks appreciated the medicinal and aphrodisiac to the amount of Omegas-3, -6, and -9, it provides properties, according to the Old Testament. It was softness, elasticity, gloss, and shine to hair. It is low imported for the first time by the Romans in Italy, spreading and absorbs well into the skin leaving it and a second time when the Arabs conquered nourished and smooth. Sicily. Pistachios flourish in hot, dry climates from the Middle East to the Mediterranean. The pistachio oil is Typical Properties Greenish-Yellow to obtained by cold pressing the seeds. This oil is mainly Appearance @ 25°C used in the food industry for its potent aroma and it Yellow Liquid has recently been considered a cosmetic ingredient. Oleic acid, % 68 - 78 Linoleic acid (C18:2), % 9 - 19 Insoluble in water Cosmetic Properties Solubility Soluble in fats and oils Pistachio oil has a very well-balanced ratio of Heavy Metals <20 ppm Omega-6 (essential fatty acids) and Omega-9, Recommended Use Level 0.5 - 5.0% responsible for its nourishing and emollient properties, resulting in silky soft and smooth skin. -

The Effect of Solvent Extraction Techniques on Fatty Acid Composition of Pistachio Oil
The Effect of solvent extraction techniques on fatty acid composition of pistachio oil Anna Abdolshahi, seyed ali Mortazavi, sahar Anna Abdolshahi, Ali Akbar Shaebani naibandi Biotechnology research center Department of Food Science and Technology Semnan University of Medical Sciences Ferdowsi university of Mashhad Semnan, Iran Mashhad, Iran [email protected] [email protected] [email protected] Abstract— Pistachio oil has important nutritional and triglycerids, LDL-cholesterol, total cholesterol and glycemic therapeutic properties because of high concentration of index (Specher, 1981). Also the increase in stability over essential fatty acids. The extraction method used to obtain oxidation of vegetable oil (Morrison and Boyd, 1981, natural compounds from row matter is critical for product IUPAC, 2007) is attributed to oleic acid. Essential fatty quality and especially for protection of their nutrition value. acids are necessary in human diet for growth maintenance This study was conducted to compare fatty acid composition of and reproduction. The linoleic acids is an essential fatty acid pistachio oil extracted by two conventional procedures: soxhlet from omega-3 group (turatti, 2000) and it is very important extraction (Sox) and maceration. Different solvents: n-Hexan for development and maintenance of the nervous system and (Hx), dichloromethane (DCM), ethyl acetate (EtAc) and the physiological functions in humans, since it reduces total ethanol (EtOH) in term of polarity index were used. The and LDL-cholesterol levels (Fagundes, 2002). highest unsaturated fatty acid content (88.493%) was obtained The extraction techniques used to obtain high aggregate by Sox with EtAc. Sox method extracted the most value compounds from natural products are critical for concentration of oleic and linolenic acids (51.99% and 0.385% respectively). -
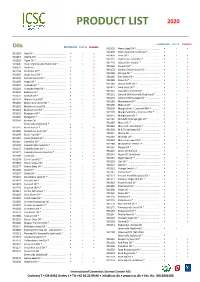
Product List 2020
PRODUCT LIST 2020 CONVENTIONAL ORGANIC STABILIZED Oils CONVENTIONAL ORGANIC STABILIZED 901199 Hemp Seed Oil * .................................. • • • 901499 Hemp Seed Oil Unrefined * ................. • • 901193 Acai Oil * ............................................. • • • 901450 Inchi Oil *............................................. • • • 901367 Alfalfa Oil* ........................................... • • • 901112 Jojoba Oil – Colorless * ........................ • • • 901228 Algae Oil * ........................................... • • • 901110 Jojoba Oil – Golden * ........................... • • • 907440 Aloe Oil (Internally Stabilized)* .......... • • 901162 Kakadu Oil * ........................................ • • • 906221 Amla Oil .............................................. • • 901152 Kalahari Melon Seed Oil * ................... • • • 901148 Andiroba Oil * ..................................... • • • 901168 Karanja Oil * ........................................ • • • 901387 Apple Seed Oil * .................................. • • • 901165 Kiwi Seed Oil * ..................................... • • • 901176 Apricot Kernel Oil * ............................. • • • 901185 Kukui Oil * ........................................... • • • 901195 Argan Oil * ........................................... • • • 901180 Lemon Seed Oil * ................................ • • • 901118 Avocado Oil * ...................................... • • • 901421 Lime Seed Oil * .................................... • • • 901218 Avocado Seed Oil * ............................. -

Rep17/Fo Joint Fao/Who Food Standards Programme
E REP17/FO JOINT FAO/WHO FOOD STANDARDS PROGRAMME CODEX ALIMENTARIUS COMMISSION Fortieth Session CICG, Geneva, Switzerland 17 – 22 July 2017 REPORT OF THE 25th SESSION OF THE CODEX COMMITTEE ON FATS AND OILS Kuala Lumpur, Malaysia 27 February – 03 March 2017 REP17/FO i TABLE OF CONTENTS Summary and Status of Work ................................................................................................................... page iii List of Acronyms ....................................................................................................................................... page v Report of the 25th Session of the Codex Committee for Fats and Oils .................................................... page 1 Paragraphs Introduction ....................................................................................................................................................... 1 Opening of the Session .............................................................................................................................. 2 - 3 Adoption of the Agenda (Agenda Item 1) .................................................................................................... 4 - 5 Matters Referred by the Codex Alimentarius Commission and other subsidiary bodies (Agenda Item 2) .......................................................................................................................................... 6 - 13 Activities of International Organizations Relevant to the Work of CCFO (Agenda Item 3) .................... -

Doctoral Thesis University of Castilla-La Mancha 2017 Adrián
Doctoral Thesis University of Castilla-La Mancha 2017 Adrián Rabadán Guerra UNIVERSIDAD DE CASTILLA-LA MANCHA ESCUELA TÉCNICA SUPERIOR DE INGENIEROS AGRÓNOMOS Y DE MONTES DEPARTAMENTO DE PRODUCCIÓN VEGETAL Y TECNOLOGÍA AGRARIA NUTS AS RAW MATERIAL FOR OIL EXTRACTION IN THE FOOD INDUSTRY: CHARACTERIZATION, PRODUCT AND PROCESS INNOVATION AND ECONOMICS Memoria presentada para aspirar al grado de Doctor por la Universidad de Castilla-La Mancha ADRIÁN RABADÁN GUERRA ALBACETE, 2017 Agradecimientos Llega el momento de dar las gracias a todas las personas que de una forma u otra han contribuido y ayudado en la realización de este trabajo. Mirando hacia atrás te das cuenta de a cuanta gente tienes que agradecer el que esta tesis sea hoy una realidad. Fundamental para el correcto desarrollo de una tesis es la adecuada elección de los directores. En este sentido he tenido la enorme suerte de contar con José Emilio Pardo, Ricardo Gómez y Manuel Álvarez, que más allá de una inmejorable guía en todo lo relacionado en la parte más técnica de tesis, han sabido, además, aportarme el apoyo personal que se necesita cuando te embarcas en un proyecto como éste. A los tres, de verdad, muchas gracias. Tengo que agradecer además su ayuda a todos mis compañeros del grupo de investigación de “Calidad, Seguridad e Higiene de Productos Agroalimentarios”, especialmente a Andrés Alvarruiz, Arturo Pardo, Eulogio López y Sergio Díaz, por todo el apoyo técnico, científico y personal. Más allá de mi propio grupo de investigación, tengo que hacer mención especial a otros profesores e investigadores que de una forma u otra han participado en este proyecto. -

Factors Influencing Storage Quality of Hazelnut Varieties
AN ABSTRACT OF THE THESIS Kais S. Ebrahem for the degree of Doctor of philosophy in Horticulture presented on October 16. 1992 Title: FACTORS INFLUENCING STORAGE QUALITY OF HAZELNUT VARIETIES Abstract approved: . ^. Dr. Daryl G.\. RichaRichardson, Professor of Horticulture This thesis research is a series of five studies dealing with various aspects of hazelnut quality: 1. Identification of kernel mold and its incidence among hazelnut varieties. 2. Seasonal development and composition of kernels. 3. Hazelnut oil composition (fatty acids and tocopherols) of seventeen varieties. 4. Hazelnut oil composition compared to 14 other nuts and oilseeds. 5. Storage and roasting effects on lipid oxidation. Barcelona, Daviana, and Ennis varieties of hazelnuts used to study white mold showed that mold incidence was highest in 1986, and much less in 1987 and 1988. Ramularia spp was the only fungus that was identified in all samples with kernel mold and was usually found at high percentages. Temperatures for drying, storage, and numbers of nuts per cluster had no significant effect on percent mold. The second part of this study measured fatty acid and vitamin E concentrations during the growing season. Samples were collected from seven varieties (Barcelona, Daviana, Ennis, Tonda Romana, Tonda Gentile della Langhe, Tombul, and Tombul Ghiaghli). Kernel oil and vitamin E (tocopherol) concentrations increased with time, while moisture decreased. Oleic acid was the major fatty acid (ca.75%) found in hazelnuts, followed by linoleic(ca.20%). Linolenic was high at the beginning of the season but then decreased to about 1% at harvest. Alpha-tocopherol was the major form of vitamin E found, composing almost 95% of total tocopherols. -

FT-IR Application for the Detection of Pistachio Oil Adulteration
ORIENTAL JOURNAL OF CHEMISTRY ISSN: 0970-020 X CODEN: OJCHEG An International Open Free Access, Peer Reviewed Research Journal 2014, Vol. 30, No. (3): Pg. 1205-1209 www.orientjchem.org FT-IR Application for the Detection of Pistachio Oil Adulteration ALI SHEIBANI*, NASER GHOTBADDINI-BAHRAMAN and FATEMEH SADEGHI Department of Chemistry, Yazd Branch, Islamic Azad University, Yazd, Iran. *Corresponding author E-mail: [email protected] http://dx.doi.org/10.13005/ojc/300335 (Received: May 10, 2014; Accepted: June 18, 2014) ABSTRACT In this work, fourier transform infrared spectroscopy (FT-IR) is used to identify and detection the adulteration of pistachio oil with cheap edible oils of corn, sunflower and soybean. For this purpose, pistachio oil was blended with cheap oils at concentration level of 10 to 60% (w/w). Then, FT-IR spectra of pure and adulterated pistachio oil samples were obtained. The fingerprints region was found to be useful in investigation of the adulteration of pistachio oil. At this region, the absorbance peaks of FT-IR decreased by increasing the adulterant amount with a linear relation that can be applied for the quality and quantity purposes. The obtained results showed that the proposed method can be considered and used as an alternative method in the detection and semi-quantization of adulteration in pistachio oil. Key word: Pistachio oil; Adulteration; FT-IR. INTRODUCTION Pistachio oil is also an expensive and important product that has a good market and can Analysis and control of the quality of edible increase the added value of pistachio cultivation. oils is an important subject in most of the countries.