Phospholipid Ratio As a Determinant of the Capacitation Interval (Interspecies Correlations/Sperm Cholesterol Efflux/Acrosome Reaction) BRIAN K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
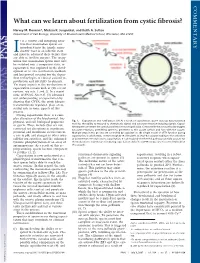
What Can We Learn About Fertilization from Cystic Fibrosis?
COMMENTARY What can we learn about fertilization from cystic fibrosis? Harvey M. Florman*, Melissa K. Jungnickel, and Keith A. Sutton Department of Cell Biology, University of Massachusetts Medical School, Worcester, MA 01655 t is a curious and intriguing situa- tion that mammalian sperm are introduced into the female repro- ductive tract in an infertile state Iand must be educated there before they are able to fertilize oocytes. The recog- nition that mammalian sperm must first be switched into a competent state, or capacitated, was exploited in the devel- opment of in vitro fertilization methods and has proved essential for the depen- dent technologies of clinical assisted re- production and infertility treatments. Yet many aspects of the mechanisms of capacitation remain unclear (for recent reviews, see refs. 1 and 2). In a recent issue of PNAS, Xu et al. (3) advanced our understanding of capacitation by showing that CFTR, the cystic fibrosis transmembrane regulator, plays an es- sential role in some aspects of this process. During capacitation there is a com- plex alteration of the biochemical, bio- physical, and cell biological properties Fig. 1. Capacitation and fertilization. (A) As a result of capacitation, sperm develop hyperactivated motility, the ability to respond to chemotactic signals and acrosome reaction-inducing signals. Capaci- of sperm. These include (but are not tated sperm penetrate the cumulus and reach the zona pellucida. Contact with the zona pellucida triggers restricted to) alterations in membrane acrosome reactions, permitting sperm to penetrate to the oocyte surface and fuse with the oocyte. potential and membrane sterol content, Multiple steps in this process are controlled by capacitation. -

Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins
International Journal of Molecular Sciences Article Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins Agnieszka Mostek *, Anna Janta , Anna Majewska and Andrzej Ciereszko Department of Gamete and Embryo Biology, Institute of Animal Reproduction and Food Research of Polish Academy of Sciences, 10-748 Olsztyn, Poland; [email protected] (A.J.); [email protected] (A.M.); [email protected] (A.C.) * Correspondence: [email protected]; Tel.: +48-89-5393134 Abstract: The ability to fertilise an egg is acquired by the mammalian sperm during the complex biochemical process called capacitation. Capacitation is accompanied by the production of reactive oxygen species (ROS), but the mechanism of redox regulation during capacitation has not been elucidated. This study aimed to verify whether capacitation coincides with reversible oxidative post-translational modifications of proteins (oxPTMs). Flow cytometry, fluorescence microscopy and Western blot analyses were used to verify the sperm capacitation process. A fluorescent gel-based redox proteomic approach allowed us to observe changes in the level of reversible oxPTMs manifested by the reduction or oxidation of susceptible cysteines in sperm proteins. Sperm capacitation was accompanied with redox modifications of 48 protein spots corresponding to 22 proteins involved in the production of ROS (SOD, DLD), playing a role in downstream redox signal transfer (GAPDHS and GST) related to the cAMP/PKA pathway (ROPN1L, SPA17), acrosome exocytosis (ACRB, sperm acrosome associated protein 9, IZUMO4), actin polymerisation (CAPZB) and hyperactivation Citation: Mostek, A.; Janta, A.; (TUBB4B, TUB1A). The results demonstrated that sperm capacitation is accompanied by altered Majewska, A.; Ciereszko, A. -

Sperm Capacitation in Surgicallyinseminated Gilts
Regional influences of the Fallopian tubes on the rate of boar sperm capacitation in surgically inseminated gilts R. H. F. Hunter, W. T. Huang and W. Holtz Institute ofAnimal Physiology and Genetics, University of Göttingen, Albrecht-Thaer-Weg 3, D-37075 Göttingen, Germany Aliquots of ejaculated boar semen containing known numbers of spermatozoa were deposited into the caudal isthmus or rostral ampulla of the Fallopian tubes of gilts at, or immediately after, ovulation to assess regional influences on the rate of capacitation. Eggs were recovered during a second intervention 4, 5, 6 or 7 h after surgical insemination and were examined by phase-contrast microscopy. Results were obtained from ten animals in each of the 4-, 5- and 6-h groups and from eight animals in the 7-h group. With two exceptions, fertilized eggs were not recovered until 6 h after insemination into the isthmus, the proportion (45.6%) being significantly greater than the corresponding figure (1.4%) for ampullary insemination (P < 0.001). Similarly, the proportion of fertilized eggs recovered 7 h after insemination into the isthmus (58.7%) was significantly greater than after ampullary insemination (21.9%; P < 0.01). Numbers of spermatozoa associated with the zona pellucida remained low in all these instances, with mean figures per egg ranging from 0.3 to 3.8. Insemination into the isthmus gave a 1\p=n-\2h advantage in fertilization compared with insemination into the ampulla. Although relative rates of sperm cell progression to the site of fertilization may have contributed to this, there is strong evidence that rates of capacitation differ significantly in the respective portions of the Fallopian tube. -

A Novel Signal Transduction Cascade in Capacitating Human Spermatozoa Characterised by a Redox-Regulated, Camp-Mediated Induction of Tyrosine Phosphorylation
Journal of Cell Science 111, 645-656 (1998) 645 Printed in Great Britain © The Company of Biologists Limited 1998 JCS3610 A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation R. J. Aitken*, D. Harkiss, W. Knox, M. Paterson and D. S. Irvine MRC Reproductive Biology Unit, 37 Chalmers Street, Edinburgh EH3 9EW, Scotland *Author for correspondence Accepted 15 December 1997: published on WWW 9 February 1998 SUMMARY Capacitation is a priming event that renders mammalian functional association was demonstrated between the redox spermatozoa responsive to signals originating from the status of human spermatozoa and their cAMP content. The cumulus-oocyte complex. The attainment of a capacitated continuous production of reactive oxygen species was also state is dependent upon an increase in tyrosine shown to be necessary for the protein kinase A-tyrosine phosphorylation and results in the acquisition of phosphorylation axis to remain functional. If the responsiveness to physiological agonists such as generation of oxidising conditions during capacitation was progesterone and ZP3. In this study we have shown that prevented with 2-mercaptoethanol, 2-deoxyglucose or the this capacitation-dependent increase in tyrosine flavoprotein inhibitor, diphenylene iodonium, then cAMP phosphorylation is controlled by a unique redox-regulated, could no longer trigger tyrosine phosphorylation. These cAMP-mediated, signal transduction cascade. Either data support a model for human sperm capacitation as a stimulation of reactive oxygen species generation or redox-regulated process, involving a unique sequence of elevation of intracellular cAMP induced increases in interactive events including reactive oxygen species phosphotyrosine expression by human spermatozoa and production, elevation of intracellular cAMP, stimulation of enhanced their responsiveness to progesterone. -

Downloaded from Bioscientifica.Com at 09/23/2021 10:31:04AM Via Free Access 478
THE RELEASE OF HYALURONIDASE FROM CAPACITATING HAMSTER SPERMATOZOA . J. ROGERS and . E. MORTON Department of Biochemistry and Biophysics, University of Hawaii, Honolulu, Hawaii 96822, U.S.A. (Received \Oth November 1972) Summary. The release of acrosomal hyaluronidase from hamster spermatozoa capacitating in test-tubes was measured. In order to do this, a colorimetrie hyaluronidase assay of increased sensitivity was developed, based upon the finding that an albumin-substrate complex was hydrolysed much more rapidly than hyaluronic acid alone. To estimate the degree of morbidity of the sperm samples, cytoplasmic lactic dehydrogenase loss into the media was monitored. This loss did not correlate with the percentage of motile spermatozoa, but was almost identical to the amount of hyaluronidase appearing in the medium when the spermatozoa were incubated in non-capacitating Tyrode's solution. Sperm hyaluronidase release, but not lactic dehydrogenase loss, was elevated by the addition of serum albumin in the presence or absence of human serum dialysate. This hyaluronidase release was magnified when heat-detoxified human serum was used to capacitate the spermatozoa. The relationship of these findings to capacitation- associated acrosome vesiculation and detachment is discussed. INTRODUCTION The cumulus oophorus, an outer barrier surrounding most mammalian ova, can be dispersed by hyaluronidase (McClean & Rowlands, 1942). This enzyme is present within the acrosomes of spermatozoa (Austin, 1960). It is released in a soluble form by conditions causing the detachment of the acrosome from the spermatozoon (Swyer, 1947). Although acrosome loss and hyaluronidase release are most commonly correlated with the loss of sperm motility (Blom, 1945; Kooner & Ludwick, 1971), acrosome-free, motile spermatozoa have been observed after sperm capacitation (Barros & Austin, 1967). -

Interaction of Sperm with the Zona Pellucida During Fertilization
Interaction of sperm with the zona pellucida during fertilization BM Gadella Department of Farm Animal Health and of Biochemistry & Cell Biology, Faculty of Veterinary Medicine, Utrecht University, Yalelaan 2, 3584 CM Utrecht, The Netherlands In order to achieve fertilization sperm cells, first need to successfully interact with the zona pellucida. To this end, the sperm surface is extensively remodeled during capacitation and the resulting sperm cells also possess hyperactivated motility. Together, this serves to mediate optimal recognition of the zona pellucida in the oviduct or after in vitro fertilization incubations (primary zona pellucida binding). When the sperm cell attaches to the zona pellucida, it will be triggered to undergo the acrosome reaction which allows the hyperactivated motile sperm cell to drill through the zona pellucida (secondary zona pellucida binding coinciding with sequential local zona pellucida digestion and rebinding). After successful zona penetration, some sperm cells may enter the perivitelline space. This delaying strategy of the oocyte allows only one sperm cell at a given time to bind and fuse with the oocyte (fertilization) and thus minimizes the risk of polyspermy. The fertilization fusion between the oocyte and the first sperm cell is immediately followed by a polyspermic fertilization block, in which the content of the oocyte's cortical granules is released into the perivitelline space. The cortical reaction blocks further sperm-oocyte fusion either by sticking at the oolemma or by the induction of a biochemical reaction of the zona pellucida (zona pellucida hardening). The cortical reaction thus blocks sperm-zona pellucida binding and/or sperm-zona pellucida penetration. This review summarizes the current understanding of sperm-zona pellucida interactions in relation to mammalian fertilization. -

Sperm Decondensation and Male Pronuclear Formation in Bovine Intracytoplasmic Sperm Injection
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School June 2020 Sperm Decondensation and Male Pronuclear Formation in Bovine Intracytoplasmic Sperm Injection Lauren Gatenby Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Animal Sciences Commons Recommended Citation Gatenby, Lauren, "Sperm Decondensation and Male Pronuclear Formation in Bovine Intracytoplasmic Sperm Injection" (2020). LSU Master's Theses. 5179. https://digitalcommons.lsu.edu/gradschool_theses/5179 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. SPERM DECONDENSATION AND MALE PRONUCLEAR FORMATION IN BOVINE INTRACYTOPLASMIC SPERM INJECTION A Thesis Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for degree of Master of Science in The School of Animal Sciences by Lauren Nicole Gatenby B.S., Louisiana State University, 2017 August 2020 ACKNOWLEDGEMENTS First and foremost, I would like to thank and express my deepest gratitude to my major professor, Dr. Kenneth Bondioli. Without his support, patience, encouragement, extensive knowledge, and mentoring abilities this would not have been possible. His contributions to both my life and education are innumerable. It has truly been a privilege to learn under his guidance and I will always be grateful. I would also like to extend a special thanks to the members of my graduate committee, Dr. Zongliang (Carl) Jiang and Dr. -
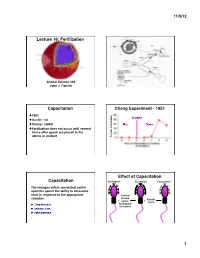
Fertilization Capacitation Chang Experiment
11/5/12! Lecture 16: Fertilization! Animal Science 434! John J. Parrish! Capacitation! Chang Experiment - 1951! 1951! Ovulation! Austin - rat! Chang - rabbit ! After! Before! Fertilization does not occur until several hours after sperm are placed in the uterus or oviduct! Percent Fertilization! Effect of Capacitation! Capacitation! Epididymal! Ejaculated! Capacitated! The changes within sperm that confer upon the sperm the ability to acrosome react in response to the appropriate Seminal! stimulus.! Plasma! Female! +! +! and/or ! Tract! Zona Pellucida! Epididymal! Secretions! Cumulus Cells! Follicular Fluid! 1! 11/5/12! DF (BSP in Bovine) Cholesterol BSP Heparin Acceptor Plasma Membrane! Plasma Membrane! Decapacitation Factor – Stabilizes plasma membrane and inhibits a premature acrosome reaction! BSP + - BSP Cholesterol H HCO3 Cholesterol Heparin Heparin Acceptor Acceptor Zp binding Zp binding Plasma Membrane! Ra: Migra=on clustering Ra: Migra=on clustering (+) sAC + H - HCO3 (+) cAMP (+) pH i PK-A (+) (-) PTK Ptyr-Ptase (+) (-) Protein Tyrosine Phosphorylaon + - BSP H HCO3 Cholesterol Heparin Extracellular Space! Acceptor Zp binding Ra: Migra=on clustering Diffusion! (+) sAC + H - + - HCO3 CO2 + H2O H2CO3 H + HCO3 (+) cAMP (+) pH i PK-A Carbonic Anhydrase! (+) (-) PTK Ptyr-Ptase (+) (-) Protein Tyrosine Phosphorylaon 2! 11/5/12! BSP 2+ Ca2+ Cholesterol Ca Heparin Acceptor Ra: Migra=on ATP ADP ATP ADP Ca2+ Ca2+ Ca2+ ATP ADP ATP ADP Ca2+ Ca2+ Acrosome Acrosome + - BSP H HCO3 Cholesterol 2+ Heparin Ca Acceptor Capacitation ! No -

Sperm Treatment Affects Capacitation Parameters and Penetration Ability of Ejaculated and Epididymal Boar Spermatozoa C
Available online at www.sciencedirect.com Theriogenology 74 (2010) 1327–1340 www.theriojournal.com Sperm treatment affects capacitation parameters and penetration ability of ejaculated and epididymal boar spermatozoa C. Matás*, M. Sansegundo, S. Ruiz, F.A. García-Vázquez, J. Gadea, R. Romar, P. Coy Department of Physiology, Faculty of Veterinary, University of Murcia, Murcia 30071, Spain Received 14 April 2010; received in revised form 2 June 2010; accepted 2 June 2010 Abstract This work was designed to study how this ability is affected by different sperm treatments routinely used for in vitro fertilization (IVF) assay. In this study, boar sperm samples from epididymal or ejaculated origin were processed by three different methods: left unwashed (NW group), washed in Dulbecco’s phosphate-buffered saline supplemented with 0.1% BSA (BSA group), and washed on a Percoll® gradient (PERCOLL group). After preparation of semen samples, changes in motility patterns were studied by CASA, calcium uptake by spectrofluorimetry, and ROS generation, spontaneous acrosome reaction, and lipid disorder by means of flow cytometry. Finally IVF assays were also performed with the different semen samples and penetrability results evaluated at 2 and 4 h post insemination (hpi). Independently of the sperm treatment, epididymal spermatozoa showed higher values of progressive motility, percentage of live cells with low lipid disorder, and penetration ability at 4 hpi than the corresponding ejaculated spermatozoa. Ejaculated spermatozoa showed higher levels of calcium uptake, ROS generation and percentage of spontaneous acrosome reaction than epididymal sperm. Regarding sperm treatments, PERCOLL group showed the highest values for some motility parameters (linearity of the curvilinear trajectory, straightness, and average path velocity/ curvilinear velocity), ROS generation and penetration ability at 2 and 4 hpi; however this same group showed the lowest values for sperm curvilinear velocity and lateral head displacement. -

Expression of Adrenergic Receptors in Mouse Preimplantation Embryos and Ovulated Oocytes
REPRODUCTIONRESEARCH Expression of adrenergic receptors in mouse preimplantation embryos and ovulated oocytes Sˇtefan Cˇ ikosˇ, Pavol Reha´k, Sonˇa Czikkova´, Jarmila Vesela´ and Juraj Koppel Institute of Animal Physiology, Slovak Academy of Sciences, Sˇolte´sovej 4, 04001 Kosˇice, Slovakia Correspondence should be addressed to Sˇ Cˇ ikosˇ; Email: [email protected] Abstract Epinephrine and norepinephrine can play an important role in basic developmental processes such as embryogenesis and morphogenesis, regulating cell proliferation, differentiation and migration. We showed that b-adrenergic receptors can mediate the effects of catecholamines on preimplantation embryos in our previous work. In the present study, we designed specific oligonucleotide primers which can distinguish among all members of the a-adrenergic receptor family, and showed (using RT-PCR) that the a2C-adrenergic receptor is transcribed in ovulated oocytes, 8- to 16-cell morulae and expanded blastocysts. We did not detect the a2C-adrenoceptor transcript in 4-cell embryos. Our immunohistochemical study showed the presence of a-2C- adrenoceptor protein in ovulated oocytes, 8- to 16- cell embryos and blastocysts, but the signal in 4-cell embryos was weak, and probably represents remaining protein of maternal origin. We did not detect any other a-adrenergic receptor in preimplantation embryos and oocytes. Exposure of mouse preimplantation embryos to the a2-adrenergic agonist UK 14 304 led to significant reduction of the embryo cell number, and the effect was dose dependent. Our results suggest that epinephrine and norepinephrine could affect the embryo development in the oviduct via adrenergic receptors directly and support the opinion that maternal stress can influence the embryo even in very early pregnancy. -

Cysteine-Rich Secretory Protein 4 Is an Inhibitor of Transient Receptor Potential M8 with a Role in Establishing Sperm Function
Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function Gerard M. Gibbsa, Gerardo Ortab, Thulasimala Reddya, Adam J. Koppersa, Pablo Martínez-Lópezb, José Luis de la Vega-Beltrànb, Jennifer C. Y. Loa, Nicholas Veldhuisc, Duangporn Jamsaia,d, Peter McIntyrec, Alberto Darszonb,1, and Moira K. O’Bryana,d,1,2 aDepartment of Anatomy and Developmental Biology, and dThe Australian Research Council Centre of Excellence in Biotechnology and Development, Monash University, VIC 3800, Australia; bDepartamento de Genética del Desarrollo y Fisiología Molecular, Instituto de Biotecnología, Universidad Nacional Autónoma de México, Cuernavaca, Morelos, México; and cDepartment of Pharmacology, University of Melbourne, Parkville 3010, Australia Edited by Ryuzo Yanagimachi, The Institute for Biogenesis Research, University of Hawaii, Honolulu, HI, and approved March 18, 2011 (received for review October 28, 2010) The cysteine-rich secretory proteins (CRISPs) are a group of four is the epididymal protein cysteine-rich secretory protein (CRISP) proteins in the mouse that are expressed abundantly in the male 4 (7, 8). reproductive tract, and to a lesser extent in other tissues. Analysis of CRISPs are a subgroup of the CRISP, antigen 5, pathogenesis- reptile CRISPs and mouse CRISP2 has shown that CRISPs can related 1 (CAP) superfamily, which is characterized by the presence of an N-terminal CAP domain (9). CRISPs are verte- regulate cellular homeostasis via ion channels. With the exception fi of the ability of CRISP2 to regulate ryanodine receptors, the in vivo brate-speci c, contain a C-terminal CRISP domain (10, 11), and targets of mammalian CRISPs function are unknown. -

An Approach to the Factors Related to Sperm Capacitation Process
y: Open log A o cc r e d s n s A López-Úbeda and Matás Andrology 2015, 4:1 Andrology-Open Access DOI: 10.4172/2167-0250.1000128 ISSN: 2167-0250 Research Article Open Access An Approach to the Factors Related to Sperm Capacitation Process Rebeca López-Úbeda and Carmen Matás* Department of Physiology, University of Murcia, Campus Mare Nostrum, 30071, Murcia, Spain Abstract This review summarizes some information about the different ways in relation to sperm capacitation. On one hand, the classical pathway that define the functional changes that occur in sperm during in vitro capacitation with special emphasis on the factors that lead to the tyrosine Phosphorylation (PY), and on the other hand, molecules and process that are involved in new mechanisms involved in this event like reactive species, especially Nitric Oxide (NO) and protein nitrosylation. Keywords: Spern capacitation; In vitro; Protein nitrosylation; Capacitation process implied several changes sequentially. Some of Phosphorylation these changes are rapid and occur at the moment of ejaculation. Others require a longer period of time in the female genital tract (in vivo) or Introduction in a medium capable of supporting this process (in vitro). All these After mating or artificial insemination, millions of sperm are processes (both rapid and slow), appear to be regulated by protein deposited in the female genital tract, of which only a small proportion is kinase A (PKA) and HCO-3, Soluble Adenylate Cyclase (SACY or able to reach the caudal portion of the isthmus (Figure 1A). This sperm sAC), and Cyclic Adenosine 3’5 ‘Monophosphate (cAMP) participate population encounters a sticky secretion of glycoprotein that modifies in this process (revised by [23]).