An Approach to the Factors Related to Sperm Capacitation Process
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
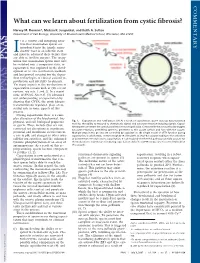
What Can We Learn About Fertilization from Cystic Fibrosis?
COMMENTARY What can we learn about fertilization from cystic fibrosis? Harvey M. Florman*, Melissa K. Jungnickel, and Keith A. Sutton Department of Cell Biology, University of Massachusetts Medical School, Worcester, MA 01655 t is a curious and intriguing situa- tion that mammalian sperm are introduced into the female repro- ductive tract in an infertile state Iand must be educated there before they are able to fertilize oocytes. The recog- nition that mammalian sperm must first be switched into a competent state, or capacitated, was exploited in the devel- opment of in vitro fertilization methods and has proved essential for the depen- dent technologies of clinical assisted re- production and infertility treatments. Yet many aspects of the mechanisms of capacitation remain unclear (for recent reviews, see refs. 1 and 2). In a recent issue of PNAS, Xu et al. (3) advanced our understanding of capacitation by showing that CFTR, the cystic fibrosis transmembrane regulator, plays an es- sential role in some aspects of this process. During capacitation there is a com- plex alteration of the biochemical, bio- physical, and cell biological properties Fig. 1. Capacitation and fertilization. (A) As a result of capacitation, sperm develop hyperactivated motility, the ability to respond to chemotactic signals and acrosome reaction-inducing signals. Capaci- of sperm. These include (but are not tated sperm penetrate the cumulus and reach the zona pellucida. Contact with the zona pellucida triggers restricted to) alterations in membrane acrosome reactions, permitting sperm to penetrate to the oocyte surface and fuse with the oocyte. potential and membrane sterol content, Multiple steps in this process are controlled by capacitation. -

Structure-Based Redesigning of Pentoxifylline Analogs Against
www.nature.com/scientificreports OPEN Structure‑based redesigning of pentoxifylline analogs against selective phosphodiesterases to modulate sperm functional competence for assisted reproductive technologies Mutyala Satish1,5, Sandhya Kumari2,5, Waghela Deeksha1, Suman Abhishek1, Kulhar Nitin1, Satish Kumar Adiga2, Padmaraj Hegde3, Jagadeesh Prasad Dasappa4, Guruprasad Kalthur2* & Eerappa Rajakumara1* Phosphodiesterase (PDE) inhibitors, such as pentoxifylline (PTX), are used as pharmacological agents to enhance sperm motility in assisted reproductive technology (ART), mainly to aid the selection of viable sperm in asthenozoospermic ejaculates and testicular spermatozoa, prior to intracytoplasmic sperm injection (ICSI). However, PTX is reported to induce premature acrosome reaction (AR) and, exert toxic efects on oocyte function and early embryo development. Additionally, in vitro binding studies as well as computational binding free energy (ΔGbind) suggest that PTX exhibits weak binding to sperm PDEs, indicating room for improvement. Aiming to reduce the adverse efects and to enhance the sperm motility, we designed and studied PTX analogues. Using structure‑guided in silico approach and by considering the physico‑chemical properties of the binding pocket of the PDEs, designed analogues of PTX. In silico assessments indicated that PTX analogues bind more tightly to PDEs and form stable complexes. Particularly, ex vivo evaluation of sperm treated with one of the PTX analogues (PTXm‑1), showed comparable benefcial efect at much lower concentration—slower -

Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins
International Journal of Molecular Sciences Article Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins Agnieszka Mostek *, Anna Janta , Anna Majewska and Andrzej Ciereszko Department of Gamete and Embryo Biology, Institute of Animal Reproduction and Food Research of Polish Academy of Sciences, 10-748 Olsztyn, Poland; [email protected] (A.J.); [email protected] (A.M.); [email protected] (A.C.) * Correspondence: [email protected]; Tel.: +48-89-5393134 Abstract: The ability to fertilise an egg is acquired by the mammalian sperm during the complex biochemical process called capacitation. Capacitation is accompanied by the production of reactive oxygen species (ROS), but the mechanism of redox regulation during capacitation has not been elucidated. This study aimed to verify whether capacitation coincides with reversible oxidative post-translational modifications of proteins (oxPTMs). Flow cytometry, fluorescence microscopy and Western blot analyses were used to verify the sperm capacitation process. A fluorescent gel-based redox proteomic approach allowed us to observe changes in the level of reversible oxPTMs manifested by the reduction or oxidation of susceptible cysteines in sperm proteins. Sperm capacitation was accompanied with redox modifications of 48 protein spots corresponding to 22 proteins involved in the production of ROS (SOD, DLD), playing a role in downstream redox signal transfer (GAPDHS and GST) related to the cAMP/PKA pathway (ROPN1L, SPA17), acrosome exocytosis (ACRB, sperm acrosome associated protein 9, IZUMO4), actin polymerisation (CAPZB) and hyperactivation Citation: Mostek, A.; Janta, A.; (TUBB4B, TUB1A). The results demonstrated that sperm capacitation is accompanied by altered Majewska, A.; Ciereszko, A. -

Chemotaxis: Communication Strategies from Bacteria to Humans
Michael Eisenbach Chemotaxis: Rina Barak Anat Bren Communication strategies Galit Cohen Ben-Lulu Fei Sun from bacteria to humans Jianshe Yan Yael Yosef Department of Biological Chemistry Tel. 972 8 934 3923 Fax. 972 8 934 4112 E-mail: [email protected] Signal transduction in bacterial chemotaxis We explore signal transduction strategies using chemotaxis of the bacteria Escherichia coli and Salmonella typhimurium as a model. Bacterial chemotaxis is a sophisticated system that integrates many different signals into a common output - a change in the direction of flagellar rotation. Our goal is to understand how CheY - a messenger protein that shuttles back and forth between the receptor supramolecular complex and the flagellar-motor supramolecular complex (Fig. 1) - brings about changes in the direction of flagellar rotation. We found that phosphorylation of CheY increases its binding to the switch protein FliM with a consequent increased probability of clockwise rotation, we identified the reciprocal binding domains on FliM and CheY, and we further found that CheY phosphorylation also Fig. 1 Simplified scheme of signal transduction in bacterial chemotaxis regulates the termination of the signal by controlling the activity of E. coli and S. typhimurium. Black arrows stand for regulated of a specific phosphatase, CheZ. Recently we investigated the protein-protein interactions. CheY is a response regulator, CheA is a correlation between the fraction of FliM molecules that are histidine kinase, and CheZ is a phosphatase. occupied by CheY and the probability of clockwise rotation. We found that this probability increases only when most of the FliM provided evidence that CheY acetylation also occurs in vivo molecules are occupied by CheY, and then the change is very and that, in the absence of Acs, chemotaxis is defective. -

Mechanisms of the Sperm Guidance, an Essential Aid for Meeting the Oocyte
430 Editorial Mechanisms of the sperm guidance, an essential aid for meeting the oocyte Raquel Lottero-Leconte*, Carlos Agustín Isidro Alonso*, Luciana Castellano, Silvina Perez Martinez Laboratory of Biology of Reproduction in Mammals, Center for Pharmacological and Botanical Studies (CEFYBO-CONICET), School of Medicine, University of Buenos Aires (UBA), Buenos Aires, Argentina *These authors contributed equally to this work. Correspondence to: Silvina Perez Martinez, Senior Investigator. Center for Pharmacological and Botanical Studies, University of Buenos Aires (UBA), School of Medicine, National Scientific and Technical Research Council-Argentina (CONICET), Paraguay 2155, 15th Floor, C1121ABG, Ciudad de Buenos Aires, Argentina. Email: [email protected]. Provenance: This is an invited Editorial commissioned by Section Editor Weijun Jiang (Nanjing Normal University, Department of Reproductive and Genetics, Institute of Laboratory Medicine, Jinling Hospital, Nanjing University School of Medicine, Nanjing, China). Comment on: De Toni L, Garolla A, Menegazzo M, et al. Heat Sensing Receptor TRPV1 Is a Mediator of Thermotaxis in Human Spermatozoa. PLoS One 2016;11:e0167622. Submitted Mar 07, 2017. Accepted for publication Mar 14, 2017. doi: 10.21037/tcr.2017.03.68 View this article at: http://dx.doi.org/10.21037/tcr.2017.03.68 In mammals, ejaculated spermatozoa must migrate into the to a temperature gradient (towards the warmer temperature) female reproductive tract in order to reach and fertilize the (Figure 1). Spermatozoa can sense both the absolute ambient oocyte (Figure 1). The number of spermatozoa that reach temperature and the temperature gradient. Previous studies the oviductal isthmus (where they attach to oviductal cells showed that, at peri-ovulation stage, there is a temperature and form the sperm reservoir) is small (1,2) and only ~10% difference between the sperm reservoir site (cooler) and the of these spermatozoa in humans become capacitated (3) fertilization site (warmer). -

SPERM THERMOTAXIS Anat Bahatand Michael Eisenbach
SPERM THERMOTAXIS Anat Bahat and Michael Eisenbach∗ Department of Biological Chemistry, The Weizmann Institute of Science, 76100 Rehovot, Israel Abstract Thermotaxis — movement directed by a temperature gradient — is a prevalent process, found from bacteria to human cells. In the case of mammalian sperm, thermotaxis appears to be an essential mechanism guiding spermatozoa, released from the cooler reservoir site, towards the warmer fertilization site. Only capacitated spermatozoa are thermotactically responsive. Thermotaxis appears to be a long-range guidance mechanism, additional to chemotaxis, which seems to be short-range and likely occurs at close proximity to the oocyte and within the cumulus mass. Both mechanisms probably have a similar function — to guide capacitated, ready-to- fertilize spermatozoa towards the oocyte. The temperature difference between the site of the sperm reservoir and the fertilization site is generated at ovulation by a temperature drop at the former. The molecular mechanism of sperm thermotaxis waits to be revealed. Keywords: Thermotaxis (sperm); Guidance (sperm); Thermosensing (sperm); Fertilization; Spermatozoa (mammalian); Female genital tract. ∗ Corresponding author. Tel: +972-8-934-3923; fax: +972-8-947-2722. E-mail address: [email protected] (M. Eisenbach). 1. Introduction A new life begins after the sperm cell (spermatozoon) meets the oocyte and initiates a series of processes that leads to sperm penetration, sperm-oocyte fusion, and zygote division. However, the chance of an incidental encounter between the gametes is very slim (Eisenbach and Tur-Kaspa, 1999; Hunter, 1993) due to a number of reasons. First, the number of ejaculated spermatozoa that reach the oviductal isthmus [where they become trapped and form a sperm reservoir (Suarez, 2002)] is small (Harper, 1982). -

Sperm Capacitation in Surgicallyinseminated Gilts
Regional influences of the Fallopian tubes on the rate of boar sperm capacitation in surgically inseminated gilts R. H. F. Hunter, W. T. Huang and W. Holtz Institute ofAnimal Physiology and Genetics, University of Göttingen, Albrecht-Thaer-Weg 3, D-37075 Göttingen, Germany Aliquots of ejaculated boar semen containing known numbers of spermatozoa were deposited into the caudal isthmus or rostral ampulla of the Fallopian tubes of gilts at, or immediately after, ovulation to assess regional influences on the rate of capacitation. Eggs were recovered during a second intervention 4, 5, 6 or 7 h after surgical insemination and were examined by phase-contrast microscopy. Results were obtained from ten animals in each of the 4-, 5- and 6-h groups and from eight animals in the 7-h group. With two exceptions, fertilized eggs were not recovered until 6 h after insemination into the isthmus, the proportion (45.6%) being significantly greater than the corresponding figure (1.4%) for ampullary insemination (P < 0.001). Similarly, the proportion of fertilized eggs recovered 7 h after insemination into the isthmus (58.7%) was significantly greater than after ampullary insemination (21.9%; P < 0.01). Numbers of spermatozoa associated with the zona pellucida remained low in all these instances, with mean figures per egg ranging from 0.3 to 3.8. Insemination into the isthmus gave a 1\p=n-\2h advantage in fertilization compared with insemination into the ampulla. Although relative rates of sperm cell progression to the site of fertilization may have contributed to this, there is strong evidence that rates of capacitation differ significantly in the respective portions of the Fallopian tube. -

An Approach to the Factors Related to Sperm Capacitation Process
y: Open log A o cc r e d s n s A López-Úbeda and Matás Andrology 2015, 4:1 Andrology-Open Access http://dx.doi.org/10.4172/2167-0250.1000128 ISSN: 2167-0250 Research Article Open Access An Approach to the Factors Related to Sperm Capacitation Process Rebeca López-Úbeda and Carmen Matás* Department of Physiology, University of Murcia, Campus Mare Nostrum, 30071, Murcia, Spain Abstract This review summarizes some information about the different ways in relation to sperm capacitation. On one hand, the classical pathway that define the functional changes that occur in sperm during in vitro capacitation with special emphasis on the factors that lead to the tyrosine Phosphorylation (PY), and on the other hand, molecules and process that are involved in new mechanisms involved in this event like reactive species, especially Nitric Oxide (NO) and protein nitrosylation. Keywords: Spern capacitation; In vitro; Protein nitrosylation; Capacitation process implied several changes sequentially. Some of Phosphorylation these changes are rapid and occur at the moment of ejaculation. Others require a longer period of time in the female genital tract (in vivo) or Introduction in a medium capable of supporting this process (in vitro). All these After mating or artificial insemination, millions of sperm are processes (both rapid and slow), appear to be regulated by protein deposited in the female genital tract, of which only a small proportion is kinase A (PKA) and HCO-3, Soluble Adenylate Cyclase (SACY or able to reach the caudal portion of the isthmus (Figure 1A). This sperm sAC), and Cyclic Adenosine 3’5 ‘Monophosphate (cAMP) participate population encounters a sticky secretion of glycoprotein that modifies in this process (revised by [23]). -

Sperm Chemotaxis Is Driven by the Slope of the Chemoattractant
bioRxiv preprint doi: https://doi.org/10.1101/148650; this version posted June 10, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license . 1 Sperm chemotaxis is driven by the slope of the chemoattractant 2 concentration field 3 Ramírez-Gómez H.V.1, Jiménez-Sabinina V.2, Tuval I.3, Velázquez-Pérez M.1, Beltrán 4 C.1, Carneiro J.4, Wood C.D.1, 5, Darszon A.1, *, Guerrero A.1, 5, *. 5 1 Departamento de Genética del Desarrollo y Fisiología Molecular, Instituto de 6 Biotecnología, Universidad Nacional Autónoma de México (UNAM), Cuernavaca, 7 Morelos, 62210, México. 8 2 Cell Biology and Biophysics Unit, European Molecular Biology Laboratory (EMBL), 9 Heidelberg, Germany. 10 3 Mediterranean Institute for Advanced Studies, IMEDEA (CSIC-UIB), Esporles, 11 Balearic Islands, Spain. 12 4 Instituto Gulbenkian de Ciência (IGC), Rua da Quinta Grande, 6 2780-156, Oeiras, 13 Portugal. 14 5 Laboratorio Nacional de Microscopía Avanzada, Instituto de Biotecnología, 15 Universidad Nacional Autónoma de México (UNAM), Cuernavaca, Morelos, 62210, 16 México. 17 * Correspondence and requests for materials should be addressed to A.G. or A.D. 18 (email: [email protected], [email protected]). 1 bioRxiv preprint doi: https://doi.org/10.1101/148650; this version posted June 10, 2017. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Sperm Chemotaxis Is Driven by the Slope of the Chemoattractant
RESEARCH ARTICLE Sperm chemotaxis is driven by the slope of the chemoattractant concentration field He´ ctor Vicente Ramı´rez-Go´ mez1, Vilma Jimenez Sabinina2, Martı´nVela´ zquez Pe´ rez1, Carmen Beltran1, Jorge Carneiro3, Christopher D Wood4, Idan Tuval5,6, Alberto Darszon1*, Ada´ n Guerrero4* 1Departamento de Gene´tica del Desarrollo y Fisiologı´a Molecular, Instituto de Biotecnologı´a, Universidad Nacional Auto´noma de Me´xico (UNAM), Cuernavaca, Mexico; 2Cell Biology and Biophysics Unit, European Molecular Biology Laboratory (EMBL), Heidelberg, Germany; 3Instituto Gulbenkian de Cieˆncia (IGC), Rua da Quinta Grande, Oeiras, Portugal; 4Laboratorio Nacional de Microscopı´a Avanzada, Instituto de Biotecnologı´a, Universidad Nacional Auto´noma de Me´xico (UNAM), Cuernavaca, Mexico; 5Mediterranean Institute for Advanced Studies, IMEDEA (CSIC-UIB), Esporles, Spain; 6Department of Physics, University of the Balearic Islands, Palma, Spain Abstract Spermatozoa of marine invertebrates are attracted to their conspecific female gamete by diffusive molecules, called chemoattractants, released from the egg investments in a process known as chemotaxis. The information from the egg chemoattractant concentration field is 2+ 2+ decoded into intracellular Ca concentration ([Ca ]i) changes that regulate the internal motors that shape the flagellum as it beats. By studying sea urchin species-specific differences in sperm chemoattractant-receptor characteristics we show that receptor density constrains the steepness of the chemoattractant concentration gradient detectable by spermatozoa. Through analyzing *For correspondence: different chemoattractant gradient forms, we demonstrate for the first time that [email protected] (AD); Strongylocentrotus purpuratus sperm are chemotactic and this response is consistent with [email protected] (AG) frequency entrainment of two coupled physiological oscillators: i) the stimulus function and ii) the 2+ Competing interests: The [Ca ]i changes. -

I Sea Urchin Sperm Chemotaxis: Individual Effects and Fertilization
Sea urchin sperm chemotaxis: individual effects and fertilization success Yasmeen H. Hussain A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Washington 2016 Reading Committee: Jeffrey A. Riffell, Chair Barbara T. Wakimoto Charles H. Muller Program Authorized to Offer Degree: Department of Biology i ©Copyright 2016 Yasmeen H. Hussain ii University of Washington Abstract Sea urchin sperm chemotaxis: individual effects and fertilization success Yasmeen H. Hussain Chair of the Supervisory Committee: Jeffrey A. Riffell Department of Biology Egg chemoattraction of conspecific sperm mediates fertilization, a critical juncture in reproduction, especially in broadcast-spawning organisms like sea urchins. In the century that sea urchin sperm chemotaxis was studied before I started my work, many discoveries were made about the variety of peptide attractants that sea urchin eggs release into the ocean environment and the molecular mechanisms of the sperm chemotactic response. However, many questions were left unanswered. Particularly, previous work has focused on the patterns that persist across populations - it is well-known that chemotaxis generally changes sperm behavior and increases recruitment to eggs. In contrast, the differences between female and male sea urchin individuals and how those differences affect the dynamics of chemoattraction and chemotaxis has not been well-studied. In this dissertation, I studied not only average behavior but also individual differences. I (i) investigated whether differences in sperm chemotactic ability between individual males translated to differences in fertilization success, (ii) measured the chemoattractant release from 1 individual females in the framework of their ability to attract sperm, and (iii) studied the behavior and physiology of sperm in specific chemoattractant gradients to understand their threshold of recruitment and fertilization-relevant response. -

A Novel Signal Transduction Cascade in Capacitating Human Spermatozoa Characterised by a Redox-Regulated, Camp-Mediated Induction of Tyrosine Phosphorylation
Journal of Cell Science 111, 645-656 (1998) 645 Printed in Great Britain © The Company of Biologists Limited 1998 JCS3610 A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation R. J. Aitken*, D. Harkiss, W. Knox, M. Paterson and D. S. Irvine MRC Reproductive Biology Unit, 37 Chalmers Street, Edinburgh EH3 9EW, Scotland *Author for correspondence Accepted 15 December 1997: published on WWW 9 February 1998 SUMMARY Capacitation is a priming event that renders mammalian functional association was demonstrated between the redox spermatozoa responsive to signals originating from the status of human spermatozoa and their cAMP content. The cumulus-oocyte complex. The attainment of a capacitated continuous production of reactive oxygen species was also state is dependent upon an increase in tyrosine shown to be necessary for the protein kinase A-tyrosine phosphorylation and results in the acquisition of phosphorylation axis to remain functional. If the responsiveness to physiological agonists such as generation of oxidising conditions during capacitation was progesterone and ZP3. In this study we have shown that prevented with 2-mercaptoethanol, 2-deoxyglucose or the this capacitation-dependent increase in tyrosine flavoprotein inhibitor, diphenylene iodonium, then cAMP phosphorylation is controlled by a unique redox-regulated, could no longer trigger tyrosine phosphorylation. These cAMP-mediated, signal transduction cascade. Either data support a model for human sperm capacitation as a stimulation of reactive oxygen species generation or redox-regulated process, involving a unique sequence of elevation of intracellular cAMP induced increases in interactive events including reactive oxygen species phosphotyrosine expression by human spermatozoa and production, elevation of intracellular cAMP, stimulation of enhanced their responsiveness to progesterone.