1. Fertilization
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Differential Sperm Motility Mediates the Sex Ratio Drive Shaping Mouse
bioRxiv preprint doi: https://doi.org/10.1101/649707; this version posted May 24, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Differential sperm motility mediates the sex ratio drive shaping mouse sex chromosome evolution Rathje CC1, Johnson EEP2, Drage D3, Patinioti C1, Silvestri G1, Affara NA2, Ialy-Radio C4, Cocquet J4, Skinner BM2,5, Ellis PJI1,5* 1 School of Biosciences, University of Kent, Canterbury, CT2 7NJ, United Kingdom 2 Department of Pathology, University of Cambridge, Tennis Court Road, Cambridge, CB2 1QP, United Kingdom 3 University Biomedical Services, University of Cambridge, Cambridge, United Kingdom 4 Department of Development, Reproduction and Cancer, INSERM, U1016, Institut Cochin, Paris, France; CNRS, UMR8104, Paris, France; Université Paris Descartes, Sorbonne Paris Cité, Faculté de Médecine, Paris, France. 5 These authors contributed equally * Corresponding author: Peter Ellis Email: P.J.I.Ellis @kent.ac.uk Tel: +44(0)1227 82 3526 KEYWORDS: Sex ratio, sex chromosomes, transmission ratio, evolution, sperm, fertilisation 1 bioRxiv preprint doi: https://doi.org/10.1101/649707; this version posted May 24, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. Summary The search for morphological or physiological differences between X- and Y-bearing mammalian sperm has provoked controversy for decades. -

In Hardening of the Zona Pellucida K
Disulfide formation in bovine zona pellucida glycoproteins during fertilization: evidence for the involvement of cystine cross-linkages in hardening of the zona pellucida K. Kwamoto, K. Ikeda, N. Yonezawa, S. Noguchi, K. Kudo, S. Hamano, M. Kuwayama and M. Nakano department ofChemistry, Faculty ofScience and 2Graduate School ofScience and Technology, Chiba University, 1-33 Yayoi-cho, Inage-ku, Chiba 263-8522, Japan; and3Animal Bio-Technology Center, Livestock Improvement Association, Tokyo, Japan The time for solubilization of the bovine zona pellucida in a hypotonic buffer containing 5% (v/v) \g=b\-mercaptoethanoland 7 mol urea l\m=-\1 increased by 10% after fertilization. Coupling with a specific fluorescent thiol probe, monobromobimane (mBBr), was markedly greater in the zona pellucida of ovarian eggs compared with fertilized eggs, indicating that the cysteine residues in the zona pellucida of unfertilized eggs are oxidized to cystines during fertilization. After endo-\g=b\-galactosidasedigestion to remove N-acetyllactosamine repeats of the carbohydrate chains, three zona pellucida glycoproteins (ZPA, ZPB and ZPC) coupled with the fluorescent bimane groups were fractionated efficiently by reverse-phase HPLC. Estimation of bimane groups in the three components and SDS-PAGE revealed that intramolecular disulfide bonds in ZPA and intra- and intermolecular disulfide bonds in ZPB were formed during fertilization, but oxidation of cysteine residues in ZPC was low. Specific proteolysis of ZPA during fertilization was also observed. These results indicate that the formation of disulfide linkages together with specific proteolysis result in the construction of a rigid zona pellucida structure, which is responsible for hardening of the zona pellucida. Introduction cross-linkages between tyrosine residues of the zona pellucida proteins formed by ovoperoxidase caused the The zona is one of the two sites at which pellucida In contrast to sea urchins (Foerder and is blocked and hardening. -

Chapter 28 *Lecture Powepoint
Chapter 28 *Lecture PowePoint The Female Reproductive System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The female reproductive system is more complex than the male system because it serves more purposes – Produces and delivers gametes – Provides nutrition and safe harbor for fetal development – Gives birth – Nourishes infant • Female system is more cyclic, and the hormones are secreted in a more complex sequence than the relatively steady secretion in the male 28-2 Sexual Differentiation • The two sexes indistinguishable for first 8 to 10 weeks of development • Female reproductive tract develops from the paramesonephric ducts – Not because of the positive action of any hormone – Because of the absence of testosterone and müllerian-inhibiting factor (MIF) 28-3 Reproductive Anatomy • Expected Learning Outcomes – Describe the structure of the ovary – Trace the female reproductive tract and describe the gross anatomy and histology of each organ – Identify the ligaments that support the female reproductive organs – Describe the blood supply to the female reproductive tract – Identify the external genitalia of the female – Describe the structure of the nonlactating breast 28-4 Sexual Differentiation • Without testosterone: – Causes mesonephric ducts to degenerate – Genital tubercle becomes the glans clitoris – Urogenital folds become the labia minora – Labioscrotal folds -

Revised Glossary for AQA GCSE Biology Student Book
Biology Glossary amino acids small molecules from which proteins are A built abiotic factor physical or non-living conditions amylase a digestive enzyme (carbohydrase) that that affect the distribution of a population in an breaks down starch ecosystem, such as light, temperature, soil pH anaerobic respiration respiration without using absorption the process by which soluble products oxygen of digestion move into the blood from the small intestine antibacterial chemicals chemicals produced by plants as a defence mechanism; the amount abstinence method of contraception whereby the produced will increase if the plant is under attack couple refrains from intercourse, particularly when an egg might be in the oviduct antibiotic e.g. penicillin; medicines that work inside the body to kill bacterial pathogens accommodation ability of the eyes to change focus antibody protein normally present in the body acid rain rain water which is made more acidic by or produced in response to an antigen, which it pollutant gases neutralises, thus producing an immune response active site the place on an enzyme where the antimicrobial resistance (AMR) an increasing substrate molecule binds problem in the twenty-first century whereby active transport in active transport, cells use energy bacteria have evolved to develop resistance against to transport substances through cell membranes antibiotics due to their overuse against a concentration gradient antiretroviral drugs drugs used to treat HIV adaptation features that organisms have to help infections; they -

Spawning & Larviculture of Bivalve Mollusks
Spawning & Larviculture of Bivalve Mollusks Grade Level: Subject Area: Time: 9-12 Aquaculture, Biology, Preparation: 30 minutes to prepare Reproduction, Anatomy Activity: 50 minute lecture (may require 1 ½ class periods) Clean-up: NA SPS (SSS): 06.04 Employ correct terminologies for animal species and conditions (e.g. sex, age, etc.) (LA.A.1.4.1-4; LA.A.2.4.4; LA.B.1.4.1-3; LA.B.2.4.1-3; LA.C.1.4.1; LA.C.2.4.1). 11.09 Develop an information file in aquaculture species (LA.A.1.4, 2.4; LA.B.1.4, 2.4; LA.C.1.4, 2.4, 3.4; LA.D.2.4; SC.D.1.4; SC.F.1.4, 2.4; SC.G.1.4, 2.4). 11.10 List and describe the major factors in the growth of aquatic fauna and flora (LA.A.1.4, 2.4; LA.B.1.4, 2.4; LA.C.1.4, 2.4, 3.4; LA.D.2.4; SC.D.1.4, SC.F.1.4, 2.4; SC.G.1.4, 2.4). 13.02 Explain how changes in water affect aquatic life (LA.A.1.4, 2.4; LA.B.2.4; LA.C.1.4, 2.4; LA.D.2.4; SC.F.1.4; SC.G.1.4). 13.03 Explain, monitor, and maintain freshwater/saltwater quality standards for the production of desirable species (LA.A.1.4, 2.4; LA.B.2.4; LA.C.1.4, 2.4; LA.D.2.4; MA>B.1.4; MA.E.1.4, 2.4, 3.4; SC.E.2.4; SC.F.2.4; SC.G.1.4). -

Chapter 9 Reproduction in Animals.Pmd
9 Reproduction in Animals MULTIPLE CHOICE QUESTIONS 1. Sets of reproductive terms are given below. Choose the set that has an incorrect combination. (a) sperm, testis, sperm duct, penis (b) menstruation, egg, oviduct, uterus (c) sperm, oviduct, egg, uterus (d) ovulation, egg, oviduct, uterus 2. In humans, the development of fertilised egg takes place in the (a) ovary (c) oviduct (b) testis (d) uterus 3. In the list of animals given below, hen is the odd one out. human being, cow, dog, hen The reason for this is (a) it undergoes internal fertilisation. (b) it is oviparous. (c) it is viviparous. (d) it undergoes external fertilisation. 4. Animals exhibiting external fertilisation produce a large number of gametes. Pick the appropriate reason from the following. (a) The animals are small in size and want to produce more offsprings. (b) Food is available in plenty in water. (c) To ensure better chance of fertilisation. (d) Water promotes production of large number of gametes. 5. Reproduction by budding takes place in (a) hydra (c) paramecium (b) amoeba (d) bacteria 6. Which of the following statements about reproduction in humans is correct? (a) Fertilisation takes place externally. 12/04/18 48 EEE XEMPLAR PROBLEMS (b) Fertilisation takes place in the testes. (c) During fertilisation egg moves towards the sperm. (d) Fertilisation takes place in the human female. 7. In human beings, after fertilisation, the structure which gets embedded in the wall of uterus is (a) ovum (c) foetus (b) embryo (d) zygote 8. Aquatic animals in which fertilisation occurs in water are said to be: (a) viviparous without fertilisation. -

Differences in Pronucleus Formation and First Cleavage Following in Vitro Fertilization Between Pig Oocytes Matured in Vivo and in Vitro J
Differences in pronucleus formation and first cleavage following in vitro fertilization between pig oocytes matured in vivo and in vitro J. Laurincik, D. Rath, and H. Niemann ^Research Institute of Animal Production, Hlohovska 2, 94992 Nitra, Slovak Republic; and zInstitut für Tierzucht und Tierverhalten (TAL) Mariensee, 31535 Neustadt, Germany To elucidate the developmental differences occurring after in vitro fertilization (IVF) of pig oocytes matured either in vitro (n = 1934) or in vivo (n = 1128), the present experiment investigated the morphological changes from penetration to the two-cell stage. Oocytes were examined every 2\p=n-\4h from 2 to 32 h after in vitro insemination to study sperm penetration, male and female pronucleus formation, synkaryosis and first cleavage. The penetration rate was significantly higher (P < 0.05) for in vivo matured oocytes (69.8%) than for in vitro matured oocytes (35.0%). Penetration of spermatozoa into the ooplasm was first recorded 6 h (in vitro matured oocytes) and 4 h (in vivo matured oocytes) after addition of the spermatozoa to the oocytes. For both in vivo and in vitro matured oocytes, 2 h were required for sperm head decondensation. However, maximum sperm head decondensation occurred 2 h later in in vitro matured oocytes. Within 6 h, 41.7 \m=+-\5.6% of the in vivo matured oocytes had completed second meiotic division, whereas only 20.8 \m=+-\6.5% of the in vitro matured oocytes reached this developmental stage (P < 0.01). For in vitro matured oocytes, male pronucleus formation was retarded 2\p=n-\4h after onset of insemination and develop- ment of the female pronucleus was enhanced compared with in vivo matured oocytes. -
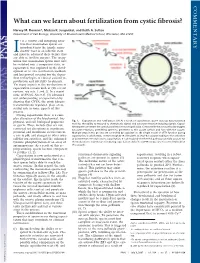
What Can We Learn About Fertilization from Cystic Fibrosis?
COMMENTARY What can we learn about fertilization from cystic fibrosis? Harvey M. Florman*, Melissa K. Jungnickel, and Keith A. Sutton Department of Cell Biology, University of Massachusetts Medical School, Worcester, MA 01655 t is a curious and intriguing situa- tion that mammalian sperm are introduced into the female repro- ductive tract in an infertile state Iand must be educated there before they are able to fertilize oocytes. The recog- nition that mammalian sperm must first be switched into a competent state, or capacitated, was exploited in the devel- opment of in vitro fertilization methods and has proved essential for the depen- dent technologies of clinical assisted re- production and infertility treatments. Yet many aspects of the mechanisms of capacitation remain unclear (for recent reviews, see refs. 1 and 2). In a recent issue of PNAS, Xu et al. (3) advanced our understanding of capacitation by showing that CFTR, the cystic fibrosis transmembrane regulator, plays an es- sential role in some aspects of this process. During capacitation there is a com- plex alteration of the biochemical, bio- physical, and cell biological properties Fig. 1. Capacitation and fertilization. (A) As a result of capacitation, sperm develop hyperactivated motility, the ability to respond to chemotactic signals and acrosome reaction-inducing signals. Capaci- of sperm. These include (but are not tated sperm penetrate the cumulus and reach the zona pellucida. Contact with the zona pellucida triggers restricted to) alterations in membrane acrosome reactions, permitting sperm to penetrate to the oocyte surface and fuse with the oocyte. potential and membrane sterol content, Multiple steps in this process are controlled by capacitation. -

An Investigation Into Pronuclear Migration in Fertilized Sea Urchin Cells
An Investigation into Pronuclear Migration in Fertilized Sea Urchin Cells Author: Sean Patrick Ruvolo Persistent link: http://hdl.handle.net/2345/bc-ir:107271 This work is posted on eScholarship@BC, Boston College University Libraries. Boston College Electronic Thesis or Dissertation, 2016 Copyright is held by the author, with all rights reserved, unless otherwise noted. Boston College Graduate School of Arts and Sciences Department of Biology AN INVESTIGATION INTO PRONUCLEAR MIGRATION IN FERTILIZED SEA URCHIN CELLS a thesis by SEAN PATRICK RUVOLO submitted in partial fulfillment of the requirements for the degree of Master of Science October 2016 © Copyright by Sean Patrick Ruvolo 2016 An Investigation into Pronuclear Migration in Fertilized Sea Urchin Cells Sean Patrick Ruvolo Advisor: David Burgess, Ph.D. After fertilization, centration of the nucleus is a necessary first step for ensuring proper cell division and embryonic development in many proliferating cells such as the sea urchin. In order for the nucleus to migrate to the cell center, the sperm aster must first capture the female pronucleus for fusion. While microtubules (MTs) are known to be necessary for centration, the precise mechanisms for both capture and centration remain undetermined. Therefore, the purpose of this research was to investigate the role of MTs in nuclear centration. Fertilized sea urchin cells were treated with the pharmacological agent, urethane (ethyl carbamate), in order to induce MT catastrophe and shrink MT asters during pronuclear capture and centration. It was discovered that proper MT length and proximity are required for pronuclear capture, since diminished asters could not interact with the female pronucleus at opposite ends of the cell. -

Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins
International Journal of Molecular Sciences Article Bull Sperm Capacitation Is Accompanied by Redox Modifications of Proteins Agnieszka Mostek *, Anna Janta , Anna Majewska and Andrzej Ciereszko Department of Gamete and Embryo Biology, Institute of Animal Reproduction and Food Research of Polish Academy of Sciences, 10-748 Olsztyn, Poland; [email protected] (A.J.); [email protected] (A.M.); [email protected] (A.C.) * Correspondence: [email protected]; Tel.: +48-89-5393134 Abstract: The ability to fertilise an egg is acquired by the mammalian sperm during the complex biochemical process called capacitation. Capacitation is accompanied by the production of reactive oxygen species (ROS), but the mechanism of redox regulation during capacitation has not been elucidated. This study aimed to verify whether capacitation coincides with reversible oxidative post-translational modifications of proteins (oxPTMs). Flow cytometry, fluorescence microscopy and Western blot analyses were used to verify the sperm capacitation process. A fluorescent gel-based redox proteomic approach allowed us to observe changes in the level of reversible oxPTMs manifested by the reduction or oxidation of susceptible cysteines in sperm proteins. Sperm capacitation was accompanied with redox modifications of 48 protein spots corresponding to 22 proteins involved in the production of ROS (SOD, DLD), playing a role in downstream redox signal transfer (GAPDHS and GST) related to the cAMP/PKA pathway (ROPN1L, SPA17), acrosome exocytosis (ACRB, sperm acrosome associated protein 9, IZUMO4), actin polymerisation (CAPZB) and hyperactivation Citation: Mostek, A.; Janta, A.; (TUBB4B, TUB1A). The results demonstrated that sperm capacitation is accompanied by altered Majewska, A.; Ciereszko, A. -

Examination of Early Cleavage an Its Importance in IVF Treatment Fancsovits P, Takacs FZ, Tothne GZ, Papp Z Urbancsek J J
Journal für Reproduktionsmedizin und Endokrinologie – Journal of Reproductive Medicine and Endocrinology – Andrologie • Embryologie & Biologie • Endokrinologie • Ethik & Recht • Genetik Gynäkologie • Kontrazeption • Psychosomatik • Reproduktionsmedizin • Urologie Examination of Early Cleavage an its Importance in IVF Treatment Fancsovits P, Takacs FZ, Tothne GZ, Papp Z Urbancsek J J. Reproduktionsmed. Endokrinol 2006; 3 (6), 367-372 www.kup.at/repromedizin Online-Datenbank mit Autoren- und Stichwortsuche Offizielles Organ: AGRBM, BRZ, DVR, DGA, DGGEF, DGRM, D·I·R, EFA, OEGRM, SRBM/DGE Indexed in EMBASE/Excerpta Medica/Scopus Krause & Pachernegg GmbH, Verlag für Medizin und Wirtschaft, A-3003 Gablitz FERRING-Symposium digitaler DVR 2021 Mission possible – personalisierte Medizin in der Reproduktionsmedizin Was kann die personalisierte Kinderwunschbehandlung in der Praxis leisten? Freuen Sie sich auf eine spannende Diskussion auf Basis aktueller Studiendaten. SAVE THE DATE 02.10.2021 Programm 12.30 – 13.20Uhr Chair: Prof. Dr. med. univ. Georg Griesinger, M.Sc. 12:30 Begrüßung Prof. Dr. med. univ. Georg Griesinger, M.Sc. & Dr. Thomas Leiers 12:35 Sind Sie bereit für die nächste Generation rFSH? Im Gespräch Prof. Dr. med. univ. Georg Griesinger, Dr. med. David S. Sauer, Dr. med. Annette Bachmann 13:05 Die smarte Erfolgsformel: Value Based Healthcare Bianca Koens 13:15 Verleihung Frederik Paulsen Preis 2021 Wir freuen uns auf Sie! Examination of Early Cleavage and its Importance in IVF Treatment P. Fancsovits, F. Z. Takács, G. Z. Tóthné, Z. Papp, J. Urbancsek Since the introduction of assisted reproduction, the number of multiple pregnancies has increased due to the high number of transferred embryos. There is an urgent need for IVF specialists to reduce the number of embryos transferred without the risk of decreasing pregnancy rates. -

Formation of the Hamster Zona Pellucida in Relation to Ovarian Differentiation and Follicular Growth M
Formation of the hamster zona pellucida in relation to ovarian differentiation and follicular growth M. C. L\l=e'\veill\l=e'\,K. D. Roberts, S. Chevalier, A. Chapdelaine and G. Bleau Departments of Obstetrics and Gynecology, Biochemistry and Medicine, University of Montreal and Maisonneuve-Rosemont Research Center, Montreal, Quebec, Canada H1T 2M4 Summary. Using an immunofluorescence technique on ovarian sections, zona\x=req-\ immunoreactive components were detected in the cytoplasm of the oocyte from the beginning of its growth, when it is surrounded by only a thin squamous follicular cell layer, up to the end of its growth. In parallel with oocyte growth, the staining intensity decreased in the ooplasm. No staining was observed in the cytoplasm of the granulosa cells during normal follicular development in adult cyclic females. However, staining of the granulosa cells was observed at some stages of follicular development in immature females. This staining was especially evident in the ovaries of immature females (22 or 26 days old) stimulated with PMSG. In addition, the staining of the granulosa cells was consistently observed in ovaries showing an abnormal histology. Increased staining of the zona at its outer and inner regions could be distinguished in normal follicles, but when staining occurred on the granulosa cells no such pattern was observed over the zona matrix. These studies indicate that the oocyte itself but not the granulosa cells elaborates the native immunogenic material of the zona pellucida. The administration of PMSG at particular stages of ovarian differentiation interferes with follicular development leading to an abnormal extracellular assembly of the zona and its degradation (phagocytosis) by the surrounding granulosa cells.