Download Thesis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Pdf/A (670.91
Phytotaxa 164 (1): 001–016 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Article PHYTOTAXA Copyright © 2014 Magnolia Press ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.164.1.1 On the monophyly of subfamily Tectarioideae (Polypodiaceae) and the phylogenetic placement of some associated fern genera FA-GUO WANG1, SAM BARRATT2, WILFREDO FALCÓN3, MICHAEL F. FAY4, SAMULI LEHTONEN5, HANNA TUOMISTO5, FU-WU XING1 & MAARTEN J. M. CHRISTENHUSZ4 1Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China. E-mail: [email protected] 2School of Biological and Biomedical Science, Durham University, Stockton Road, Durham, DH1 3LE, United Kingdom. 3Institute of Evolutionary Biology and Environmental Studies, University of Zurich, Winterthurerstrasse 190, 8075 Zurich, Switzerland. 4Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 4DS, United Kingdom. E-mail: [email protected] (author for correspondence) 5Department of Biology, University of Turku, FI-20014 Turku, Finland. Abstract The fern genus Tectaria has generally been placed in the family Tectariaceae or in subfamily Tectarioideae (placed in Dennstaedtiaceae, Dryopteridaceae or Polypodiaceae), both of which have been variously circumscribed in the past. Here we study for the first time the phylogenetic relationships of the associated genera Hypoderris (endemic to the Caribbean), Cionidium (endemic to New Caledonia) and Pseudotectaria (endemic to Madagascar and Comoros) using DNA sequence data. Based on a broad sampling of 72 species of eupolypods I (= Polypodiaceae sensu lato) and three plastid DNA regions (atpA, rbcL and the trnL-F intergenic spacer) we were able to place the three previously unsampled genera. -

The New York Botanical Garden
Vol. XV DECEMBER, 1914 No. 180 JOURNAL The New York Botanical Garden EDITOR ARLOW BURDETTE STOUT Director of the Laboratories CONTENTS PAGE Index to Volumes I-XV »33 PUBLISHED FOR THE GARDEN AT 41 NORTH QUBKN STRHBT, LANCASTER, PA. THI NEW ERA PRINTING COMPANY OFFICERS 1914 PRESIDENT—W. GILMAN THOMPSON „ „ _ i ANDREW CARNEGIE VICE PRESIDENTS J FRANCIS LYNDE STETSON TREASURER—JAMES A. SCRYMSER SECRETARY—N. L. BRITTON BOARD OF- MANAGERS 1. ELECTED MANAGERS Term expires January, 1915 N. L. BRITTON W. J. MATHESON ANDREW CARNEGIE W GILMAN THOMPSON LEWIS RUTHERFORD MORRIS Term expire January. 1916 THOMAS H. HUBBARD FRANCIS LYNDE STETSON GEORGE W. PERKINS MVLES TIERNEY LOUIS C. TIFFANY Term expire* January, 1917 EDWARD D. ADAMS JAMES A. SCRYMSER ROBERT W. DE FOREST HENRY W. DE FOREST J. P. MORGAN DANIEL GUGGENHEIM 2. EX-OFFICIO MANAGERS THE MAYOR OP THE CITY OF NEW YORK HON. JOHN PURROY MITCHEL THE PRESIDENT OP THE DEPARTMENT OP PUBLIC PARES HON. GEORGE CABOT WARD 3. SCIENTIFIC DIRECTORS PROF. H. H. RUSBY. Chairman EUGENE P. BICKNELL PROF. WILLIAM J. GIES DR. NICHOLAS MURRAY BUTLER PROF. R. A. HARPER THOMAS W. CHURCHILL PROF. JAMES F. KEMP PROF. FREDERIC S. LEE GARDEN STAFF DR. N. L. BRITTON, Director-in-Chief (Development, Administration) DR. W. A. MURRILL, Assistant Director (Administration) DR. JOHN K. SMALL, Head Curator of the Museums (Flowering Plants) DR. P. A. RYDBERG, Curator (Flowering Plants) DR. MARSHALL A. HOWE, Curator (Flowerless Plants) DR. FRED J. SEAVER, Curator (Flowerless Plants) ROBERT S. WILLIAMS, Administrative Assistant PERCY WILSON, Associate Curator DR. FRANCIS W. PENNELL, Associate Curator GEORGE V. -

A Rapid Biological Assessment of the Upper Palumeu River Watershed (Grensgebergte and Kasikasima) of Southeastern Suriname
Rapid Assessment Program A Rapid Biological Assessment of the Upper Palumeu River Watershed (Grensgebergte and Kasikasima) of Southeastern Suriname Editors: Leeanne E. Alonso and Trond H. Larsen 67 CONSERVATION INTERNATIONAL - SURINAME CONSERVATION INTERNATIONAL GLOBAL WILDLIFE CONSERVATION ANTON DE KOM UNIVERSITY OF SURINAME THE SURINAME FOREST SERVICE (LBB) NATURE CONSERVATION DIVISION (NB) FOUNDATION FOR FOREST MANAGEMENT AND PRODUCTION CONTROL (SBB) SURINAME CONSERVATION FOUNDATION THE HARBERS FAMILY FOUNDATION Rapid Assessment Program A Rapid Biological Assessment of the Upper Palumeu River Watershed RAP (Grensgebergte and Kasikasima) of Southeastern Suriname Bulletin of Biological Assessment 67 Editors: Leeanne E. Alonso and Trond H. Larsen CONSERVATION INTERNATIONAL - SURINAME CONSERVATION INTERNATIONAL GLOBAL WILDLIFE CONSERVATION ANTON DE KOM UNIVERSITY OF SURINAME THE SURINAME FOREST SERVICE (LBB) NATURE CONSERVATION DIVISION (NB) FOUNDATION FOR FOREST MANAGEMENT AND PRODUCTION CONTROL (SBB) SURINAME CONSERVATION FOUNDATION THE HARBERS FAMILY FOUNDATION The RAP Bulletin of Biological Assessment is published by: Conservation International 2011 Crystal Drive, Suite 500 Arlington, VA USA 22202 Tel : +1 703-341-2400 www.conservation.org Cover photos: The RAP team surveyed the Grensgebergte Mountains and Upper Palumeu Watershed, as well as the Middle Palumeu River and Kasikasima Mountains visible here. Freshwater resources originating here are vital for all of Suriname. (T. Larsen) Glass frogs (Hyalinobatrachium cf. taylori) lay their -

Biogeographical Patterns of Species Richness, Range Size And
Biogeographical patterns of species richness, range size and phylogenetic diversity of ferns along elevational-latitudinal gradients in the tropics and its transition zone Kumulative Dissertation zur Erlangung als Doktorgrades der Naturwissenschaften (Dr.rer.nat.) dem Fachbereich Geographie der Philipps-Universität Marburg vorgelegt von Adriana Carolina Hernández Rojas aus Xalapa, Veracruz, Mexiko Marburg/Lahn, September 2020 Vom Fachbereich Geographie der Philipps-Universität Marburg als Dissertation am 10.09.2020 angenommen. Erstgutachter: Prof. Dr. Georg Miehe (Marburg) Zweitgutachterin: Prof. Dr. Maaike Bader (Marburg) Tag der mündlichen Prüfung: 27.10.2020 “An overwhelming body of evidence supports the conclusion that every organism alive today and all those who have ever lived are members of a shared heritage that extends back to the origin of life 3.8 billion years ago”. This sentence is an invitation to reflect about our non- independence as a living beins. We are part of something bigger! "Eine überwältigende Anzahl von Beweisen stützt die Schlussfolgerung, dass jeder heute lebende Organismus und alle, die jemals gelebt haben, Mitglieder eines gemeinsamen Erbes sind, das bis zum Ursprung des Lebens vor 3,8 Milliarden Jahren zurückreicht." Dieser Satz ist eine Einladung, über unsere Nichtunabhängigkeit als Lebende Wesen zu reflektieren. Wir sind Teil von etwas Größerem! PREFACE All doors were opened to start this travel, beginning for the many magical pristine forest of Ecuador, Sierra de Juárez Oaxaca and los Tuxtlas in Veracruz, some of the most biodiverse zones in the planet, were I had the honor to put my feet, contemplate their beauty and perfection and work in their mystical forest. It was a dream into reality! The collaboration with the German counterpart started at the beginning of my academic career and I never imagine that this will be continued to bring this research that summarizes the efforts of many researchers that worked hardly in the overwhelming and incredible biodiverse tropics. -

An Integrated Phylogeographic Analysis of the Bantu Migration
AN INTEGRATED PHYLOGEOGRAPHIC ANALYSIS OF THE BANTU MIGRATION by Colby Tyler Ford A dissertation submitted to the faculty of The University of North Carolina at Charlotte in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Bioinformatics and Computational Biology Charlotte 2018 Approved by: Dr. Daniel Janies Dr. Xinghua Shi Dr. Anthony Fodor Dr. Mirsad Hadzikadic Dr. Matthew Parrow ii ©2018 Colby Tyler Ford ALL RIGHTS RESERVED iii ABSTRACT COLBY TYLER FORD. An Integrated Phylogeographic Analysis of the Bantu Migration. (Under the direction of DR. DANIEL JANIES) \Bantu" is a term used to describe lineages of people in around 600 different ethnic groups on the African continent ranging from modern-day Cameroon to South Africa. The migration of the Bantu people, which occurred around 3,000 years ago, was influ- ential in spreading culture, language, and genetic traits and helped to shape human diversity on the continent. Research in the 1970s was completed to geographically divide the Bantu languages into 16 zones now known as \Guthrie zones" [25]. Researchers have postulated the migratory pattern of the Bantu people by exam- ining cultural information, linguistic traits, or small genetic datasets. These studies offer differing results due to variations in the data type used. Here, an assessment of the Bantu migration is made using a large dataset of combined cultural data and genetic (Y-chromosomal and mitochondrial) data. One working hypothesis is that the Bantu expansion can be characterized by a primary split in lineages, which occurred early on and prior to the population spread- ing south through what is now called the Congolese forest (i.e. -

Fern Classification
16 Fern classification ALAN R. SMITH, KATHLEEN M. PRYER, ERIC SCHUETTPELZ, PETRA KORALL, HARALD SCHNEIDER, AND PAUL G. WOLF 16.1 Introduction and historical summary / Over the past 70 years, many fern classifications, nearly all based on morphology, most explicitly or implicitly phylogenetic, have been proposed. The most complete and commonly used classifications, some intended primar• ily as herbarium (filing) schemes, are summarized in Table 16.1, and include: Christensen (1938), Copeland (1947), Holttum (1947, 1949), Nayar (1970), Bierhorst (1971), Crabbe et al. (1975), Pichi Sermolli (1977), Ching (1978), Tryon and Tryon (1982), Kramer (in Kubitzki, 1990), Hennipman (1996), and Stevenson and Loconte (1996). Other classifications or trees implying relationships, some with a regional focus, include Bower (1926), Ching (1940), Dickason (1946), Wagner (1969), Tagawa and Iwatsuki (1972), Holttum (1973), and Mickel (1974). Tryon (1952) and Pichi Sermolli (1973) reviewed and reproduced many of these and still earlier classifica• tions, and Pichi Sermolli (1970, 1981, 1982, 1986) also summarized information on family names of ferns. Smith (1996) provided a summary and discussion of recent classifications. With the advent of cladistic methods and molecular sequencing techniques, there has been an increased interest in classifications reflecting evolutionary relationships. Phylogenetic studies robustly support a basal dichotomy within vascular plants, separating the lycophytes (less than 1 % of extant vascular plants) from the euphyllophytes (Figure 16.l; Raubeson and Jansen, 1992, Kenrick and Crane, 1997; Pryer et al., 2001a, 2004a, 2004b; Qiu et al., 2006). Living euphyl• lophytes, in turn, comprise two major clades: spermatophytes (seed plants), which are in excess of 260 000 species (Thorne, 2002; Scotland and Wortley, Biology and Evolution of Ferns and Lycopliytes, ed. -

Alansmia, a New Genus of Grammitid Ferns (Polypodiaceae) Segregated from Terpsichore
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Alansmia, a new genus of grammitid ferns (Polypodiaceae) segregated from Terpsichore 1 2,3 4 MICHAEL KESSLER ,ANA LAURA MOGUEL VELÁZQUEZ ,MICHAEL SUNDUE , 5 AND PAULO H. LABIAK 1 Systematic Botany, University of Zurich, Zollikerstrasse 107, CH-8008, Zurich, Switzerland; e-mail: [email protected] 2 Department of Systematic Botany, Albrecht-von-Haller-Institute of Plant Sciences, Georg-August- University, Untere Karspüle 2, 37073, Göttingen, Germany 3 Present Address: Pfefferackerstr. 22, 45894, Gelsenkirchen, Germany; e-mail: [email protected] 4 The New York Botanical Garden, 200th St. and Southern Blvd., Bronx, NY 10458, USA; e-mail: [email protected] 5 Departamento de Botânica, Universidade Federal do Paraná, Caixa Postal 19031( 81531-980, Curitiba, PR, Brazil; e-mail: [email protected] Abstract. Alansmia, a new genus of grammitid ferns is described and combinations are made for the 26 species known to belong to it. Alansmia is supported by five morphological synapomorphies: setae present on the rhizomes, cells of the rhizome scales turgid, both surfaces of the rhizome scales ciliate, laminae membranaceous, and sporangial capsules setose. Other diagnostic characters include pendent fronds with indeterminate growth, concolorous, orange to castaneous rhizome scales with ciliate or sometimes glandular margins, hydathodes often cretaceous, and setae simple, paired or stellate. The group also exhibits the uncommon characteristic of producing both trilete and apparently monolete spores, sometimes on the same plant. New combinations are made for Alansmia alfaroi, A. bradeana, A. canescens, A. concinna, A. -

DRAFT Fern and Lycophyte Genera of BELIZE DRAFT 1
DRAFT Fern and Lycophyte Genera of BELIZE DRAFT 1 Sally M. Chambers1, Bruce K. Holst1, Ella Baron2, David Amaya2& Marvin Paredes2 1Marie Selby Botanical Gardens, 2 Ian Anderson’s Caves Branch Botanical Garden © Marie Selby Botanical Gardens [[email protected]], Ian Anderson’s Caves Branch Botanical Garden ([email protected]). Photos by D. Amaya (DA), E. Baron (EB), B. Holst (BH), J. Meerman (JM), R. Moran (RM), , P. Nelson (PN), M. Sundue (MS) Support from the Marie Selby Botanical Gardens, Ian Anderson’s Caves Branch Botanical Garden, Environmental Resource Institute - University of Belize [fieldguides.fieldmusuem.org] [ guide’s number ###] version 1 12/2017 There are 33 known fern genera in Belize, growing as epiphytes on trees and shrubs, on rocks (lithophytes), or on the ground and climbing in to the canopy (hemiepiphytes). These genera are distinguished based on frond shape, sori, growth habit, and rhizome characteristics. Definitions of these features, and the different characteristics they exhibit may be found at the end of this guide. Ferns and lycophytes (Phlegmariurus is the only known epiphytic lycophyte genus in Belize) can be distinguished by the location in which the sori are produced (facing the stem in lycophytes, away from the stem in ferns), the presence of relatively small leaves (microphylls) in lycophytes, and differences in xylem development. This guide provides brief descriptions for each genus, along with photographs displaying critical characteristics for identification. The number of species for each genus found within Belize is provided in parentheses following the genus name. Species names are provided in each figure caption, denoted by the first letter of the focal genus and the specific epithet. -

Genetic Structuring, Dispersal and Taxonomy of the High-Alpine Populations of the Geranium Arabicum/Kilimandscharicum Complex in Tropical Eastern Africa
RESEARCH ARTICLE Genetic structuring, dispersal and taxonomy of the high-alpine populations of the Geranium arabicum/kilimandscharicum complex in tropical eastern Africa Tigist Wondimu1,2*, Abel Gizaw1,2, Felly M. Tusiime2,3, Catherine A. Masao2,4, Ahmed A. Abdi2,5, Yan Hou2, Sileshi Nemomissa1, Christian Brochmann2 1 Department of Plant Biology & Biodiversity Management, College of Natural Sciences, Addis Ababa a1111111111 University, Addis Ababa, Ethiopia, 2 Natural History Museum, University of Oslo, Blindern, Oslo, Norway, a1111111111 3 Department of Forestry and Tourism, School of Forestry, Geographical and Environmental Sciences, a1111111111 Makerere University, Kampala, Uganda, 4 University of Dar es Salaam, Institute of Resource Assessment, a1111111111 Dar es Salaam, Tanzania, 5 National Museums of Kenya, Nairobi, Kenya a1111111111 * [email protected], [email protected] Abstract OPEN ACCESS The scattered eastern African high mountains harbor a renowned and highly endemic flora, Citation: Wondimu T, Gizaw A, Tusiime FM, Masao but the taxonomy and phylogeographic history of many plant groups are still insufficiently CA, Abdi AA, Hou Y, et al. (2017) Genetic structuring, dispersal and taxonomy of the high- known. The high-alpine populations of the Geranium arabicum/kilimandscharicum complex alpine populations of the Geranium arabicum/ present intricate morphological variation and have recently been suggested to comprise two kilimandscharicum complex in tropical eastern new endemic taxa. Here we aim to contribute to a clarification of the taxonomy of these pop- Africa. PLoS ONE 12(5): e0178208. https://doi.org/ 10.1371/journal.pone.0178208 ulations by analyzing genetic (AFLP) variation in range-wide high-alpine samples, and we address whether hybridization has contributed to taxonomic problems. -
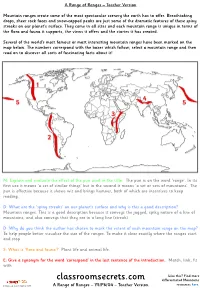
Classroomsecrets.Com Differentiated Mountains
A Range of Ranges – Teacher Version Mountain ranges create some of the most spectacular scenery the earth has to offer. Breathtaking drops, sheer rock faces and snow-capped peaks are just some of the dramatic features of these spiny streaks on our planet’s surface. They come in all sizes and each mountain range is unique in terms of the flora and fauna it supports, the views it offers and the stories it has created. Several of the world’s most famous or most interesting mountain ranges have been marked on the map below. The numbers correspond with the boxes which follow; select a mountain range and then read on to discover all sorts of fascinating facts about it! M: Explain and evaluate the effect of the pun used in the title. The pun is on the word ‘range’. In its first use it means ‘a set of similar things’ but in the second it means ‘a set or sets of mountains’. The pun is effective because it shows wit and brings humour, both of which are incentives to keep reading. D: What are the ‘spiny streaks’ on our planet’s surface and why is this a good description? Mountain ranges. This is a good description because it conveys the jagged, spiky nature of a line of mountains, and also conveys that they are in a long line (streak). D: Why do you think the author has chosen to mark the extent of each mountain range on the map? To help people better visualise the size of the ranges. To make it clear exactly where the ranges start and stop. -

Unraveling the Causal Links Between Ecosystem Productivity Measures and Species Richness Using Terrestrial Ferns in Ecuador
GÖTTINGER ZENTRUM FÜR BIODIVERSITÄT FORSCHUNG UND ÖKOLOGIE - GÖTTINGEN CENTRE FOR BIODIVERSITY AND ECOLOGY - Unraveling the causal links between ecosystem productivity measures and species richness using terrestrial ferns in Ecuador Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultäten der Georg-August-Universität Göttingen vorgelegt von Laura Inés Salazar Cotugno aus Quito, Ecuador Göttingen, Oktober, 2012 Referent: Prof. Dr. Christoph Leuschner Korreferent: PD. Dr. Michael Kessler Tag der mündlichen Prüfung: A mis queridos Lili y Diego Abstract This work focuses on the relationship between terrestrial fern species richness and productivity, and on the fern nutrient availability along a tropical elevational gradient in Ecuador. During three yearly field phases between 2009 and 2011, field work was carried out at eight elevations (500 m to 4000 m) on the eastern Andean slope in Ecuador. Diversity, biomass, productivity and leaf functional traits of terrestrial ferns were recorded in three permanent plots of 400m2 each per elevation. In Chapter 1, I outlined the general purpose of this dissertation, as well as general concepts. In Chapter 2, an alternative to measure air humidity is proposed. In Chapter 3, a total of 91 terrestrial fern species, in 32 genera and 18 families are reported. Hump-shaped patterns along the elevational gradient with a peak at mid elevations adequately described fern species richness, which confirmed that fern diversity is primarily driven by energy-related variables, and that especially low annual variability of these factors favors species rich fern communities. The main results of Chapter 4 showed that along the elevational gradient, terrestrial fern species richness was only weakly related to measures of ecosystem productivity, and more closely to the productivity of the terrestrial fern assemblages as such, which appeared to be determined by an increase in the number of fern individuals and by niche availability.