Improved Response of Growth Hormone to Growth Hormone
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands
The Activation of the Glucagon-Like Peptide-1 (GLP-1) Receptor by Peptide and Non-Peptide Ligands Clare Louise Wishart Submitted in accordance with the requirements for the degree of Doctor of Philosophy of Science University of Leeds School of Biomedical Sciences Faculty of Biological Sciences September 2013 I Intellectual Property and Publication Statements The candidate confirms that the work submitted is her own and that appropriate credit has been given where reference has been made to the work of others. This copy has been supplied on the understanding that it is copyright material and that no quotation from the thesis may be published without proper acknowledgement. The right of Clare Louise Wishart to be identified as Author of this work has been asserted by her in accordance with the Copyright, Designs and Patents Act 1988. © 2013 The University of Leeds and Clare Louise Wishart. II Acknowledgments Firstly I would like to offer my sincerest thanks and gratitude to my supervisor, Dr. Dan Donnelly, who has been nothing but encouraging and engaging from day one. I have thoroughly enjoyed every moment of working alongside him and learning from his guidance and wisdom. My thanks go to my academic assessor Professor Paul Milner whom I have known for several years, and during my time at the University of Leeds he has offered me invaluable advice and inspiration. Additionally I would like to thank my academic project advisor Dr. Michael Harrison for his friendship, help and advice. I would like to thank Dr. Rosalind Mann and Dr. Elsayed Nasr for welcoming me into the lab as a new PhD student and sharing their experimental techniques with me, these techniques have helped me no end in my time as a research student. -

List of Approved Ndas for Biological Products That Were Deemed to Be Blas on March 23, 2020
List of Approved NDAs for Biological Products That Were Deemed to be BLAs on March 23, 2020 On March 23, 2020, an approved application for a biological product under section 505 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) was deemed to be a license for the biological product under section 351 of the Public Health Service Act (PHS Act) (see section 7002(e)(4)(A) of the Biologics Price Competition and Innovation Act of 2009). To enhance transparency and facilitate planning for the March 23, 2020, transition date, FDA compiled a preliminary list of approved applications for biological products under the FD&C Act that were listed in FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations (the Orange Book) and that would be affected by this transition provision. FDA posted this list on the FDA website in December 2018, and periodically updated this list before the March 23, 2020, transition date. The September 2019 update to this preliminary list added certain administratively closed applications related to approved applications for biological products that were on the December 2018 version of this list. The January 2020 update to the preliminary list reflected a change to the definition of “biological product” made by the Further Consolidated Appropriations Act, 2020, which was enacted on December 20, 2019. Section 605 of this Act further amended the definition of a “biological product” in section 351(i) of the PHS Act to remove the parenthetical “(except any chemically synthesized polypeptide)” from the statutory category of “protein.” FDA has provided below a list of each approved application for a biological product under the FD&C Act that was deemed to be a license (i.e., an approved biologics license application (BLA)) for the biological product on March 23, 2020. -

Corticorelin Acetate, a Synthetic Corticotropin-Releasing Factor with Preclinical Antitumor Activity, Alone and with Bevacizumab, Against Human Brain Tumor Models
ANTICANCER RESEARCH 30: 5037-5042 (2010) Corticorelin Acetate, a Synthetic Corticotropin-releasing Factor with Preclinical Antitumor Activity, alone and with Bevacizumab, against Human Brain Tumor Models IDOIA GAMEZ1, ROBERT P. RYAN1 and STEPHEN T. KEIR2 1Celtic Pharmaceutical Development Services America, Inc., New York, NY 10022, U.S.A.; 2The Preston Robert Tisch Brain Tumor Center, Duke University, Durham, NC 27710, U.S.A. Abstract. Background: Corticorelin acetate (CrA) is a brain tumors. The cause of PBE is believed to result in part synthetic form of corticotropin-releasing factor that is from the leakage of edematous brain tumor fluid from currently undergoing clinical trials in the treatment of abnormal tumor vasculature into the surrounding tissue (1, peritumoral brain edema (PBE). This study preclinically 2). The mechanism(s) of action by which CrA exerts its investigated its potential as an antitumor agent against beneficial effect in the therapy of PBE has not been human brain tumor xenografts. Materials and Methods: The elucidated, but two factors appear to be relevant: decreased in vivo efficacy of CrA as a single agent and in combination vascular leakage and preserved integrity of the endothelial with the antiangiogenic agent, bevacizumab, was examined cells, which ultimately helps to maintain the blood-brain in three different patient-derived human brain tumor barrier (3, 4). xenografts implanted orthotopically (intracranially) or CrA appears to mediate its activity through two subtypes subcutaneously in athymic mice. Results: CrA significantly of G-protein coupled receptors: CRF receptor-1 (CRFR1) increased the lifespan of mice implanted orthotopically with and CRF receptor-2 (CRFR2) (5). These CRFRs are widely two different pediatric brain tumor xenograft models. -

BCBSVT Specialty Drug List Effective 2021.07.01.Xlsx
Effective Date: 07/01/2021 SPECIALTY DRUG LIST Revised Date: 05/07/2021 DOSAGE EXCLUDED ON NATIONAL DRUG CLASS DRUG NAME GENERIC NAME FORM PERFORMANCE FORMULARY ANEMIA ARANESP SOLN DARBEPOETIN ALFA SOLN INJ ANEMIA ARANESP SOSY DARBEPOETIN ALFA SOLN PREFILLED SYRINGE ANEMIA EPOGEN SOLN EPOETIN ALFA INJ X ANEMIA PROCRIT SOLN EPOETIN ALFA INJ X ANEMIA REBLOZYL SOLR LUSPATERCEPT-AAMT FOR SUBCUTANEOUS INJ ANEMIA RETACRIT SOLN EPOETIN ALFA-EPBX INJ ANTI-GOUT AGENT KRYSTEXXA SOLN PEGLOTICASE INJ (FOR IV INFUSION) ANTI-INFECTIVE PREVYMIS SOLN LETERMOVIR IV SOLN ANTI-INFECTIVE PREVYMIS TABS LETERMOVIR TAB ASTHMA CINQAIR SOLN RESLIZUMAB IV INFUSION SOLN ASTHMA FASENRA SOSY BENRALIZUMAB SUBCUTANEOUS SOLN PREFILLED SYRINGE ASTHMA FASENRA PEN SOAJ BENRALIZUMAB SUBCUTANEOUS SOLN AUTO-INJECTOR ASTHMA NUCALA SOAJ MEPOLIZUMAB SUBCUTANEOUS SOLUTION AUTO-INJECTOR ASTHMA NUCALA SOLR MEPOLIZUMAB FOR INJ ASTHMA NUCALA SOSY MEPOLIZUMAB SUBCUTANEOUS SOLUTION PREF SYRINGE ASTHMA XOLAIR SOLR OMALIZUMAB FOR INJ ASTHMA XOLAIR SOSY OMALIZUMAB SUBCUTANEOUS SOLN PREFILLED SYRINGE CARDIOVASCULAR VYNDAMAX CAPS TAFAMIDIS CAP CARDIOVASCULAR VYNDAQEL CAPS TAFAMIDIS MEGLUMINE (CARDIAC) CAP CENTRAL NERVOUS SYSTEM AGENTS AUSTEDO TABS DEUTETRABENAZINE TAB CENTRAL NERVOUS SYSTEM AGENTS ENSPRYNG SOSY SATRALIZUMAB-MWGE SUBCUTANEOUS SOLN PREF SYRINGE CENTRAL NERVOUS SYSTEM AGENTS HETLIOZ CAPS TASIMELTEON CAPSULE CENTRAL NERVOUS SYSTEM AGENTS HETLIOZ LQ SUSP TASIMELTEON ORAL SUSP CHEMOTHERAPY PROTECTANT AMIFOSTINE SOLR AMIFOSTINE CRYSTALLINE FOR INJ CHEMOTHERAPY PROTECTANT ELITEK -

Preferred Drug List (Formulary)
January 2020 Molina Healthcare of Michigan Preferred Drug List (Formulary) 1 Molina Healthcare of Michigan Preferred Drug List (Formulary) (01/01/2020) INTRODUCTION ..........................................................................................................................................................................................................................................5 PREFACE .....................................................................................................................................................................................................................................................5 PHARMACY AND THERAPEUTICS (P&T) COMMITTEE ..........................................................................................................................................................................5 DRUG LIST PRODUCT DESCRIPTIONS ...................................................................................................................................................................................................5 GENERIC SUBSTITUTION ..........................................................................................................................................................................................................................5 PLAN DESIGN .............................................................................................................................................................................................................................................6 -

September 2017 ~ Resource #330909
−This Clinical Resource gives subscribers additional insight related to the Recommendations published in− September 2017 ~ Resource #330909 Medications Stored in the Refrigerator (Information below comes from current U.S. and Canadian product labeling and is current as of date of publication) Proper medication storage is important to ensure medication shelf life until the manufacturer expiration date and to reduce waste. Many meds are recommended to be stored at controlled-room temperature. However, several meds require storage in the refrigerator or freezer to ensure stability. See our toolbox, Medication Storage: Maintaining the Cold Chain, for helpful storage tips and other resources. Though most meds requiring storage at temperatures colder than room temperature should be stored in the refrigerator, expect to see a few meds require storage in the freezer. Some examples of medications requiring frozen storage conditions include: anthrax immune globulin (Anthrasil [U.S. only]), carmustine wafer (Gliadel [U.S. only]), cholera (live) vaccine (Vaxchora), dinoprostone vaginal insert (Cervidil), dinoprostone vaginal suppository (Prostin E2 [U.S.]), varicella vaccine (Varivax [U.S.]; Varivax III [Canada] can be stored in the refrigerator or freezer), zoster vaccine (Zostavax [U.S.]; Zostavax II [Canada] can be stored in the refrigerator or freezer). Use the list below to help identify medications requiring refrigerator storage and become familiar with acceptable temperature excursions from recommended storage conditions. Abbreviations: RT = room temperature Abaloparatide (Tymlos [U.S.]) Aflibercept (Eylea) Amphotericin B (Abelcet, Fungizone) • Once open, may store at RT (68°F to 77°F • May store at RT (77°F [25°C]) for up to Anakinra (Kineret) [20°C to 25°C]) for up to 30 days. -

Michigan Department of Health and Human Services Practitioner and Medical Clinic Fee Schedule Revised: 04/06/2016 January - 2016
Michigan Department of Health and Human Services Practitioner and Medical Clinic Fee Schedule Revised: 04/06/2016 January - 2016 Code Short Description Modifier Age Range Non Fac Fee Fac Fee Effective Date** 0054T Bone Srgry Cmptr Fluor Image NA $84.39 0055T Bone Srgry Cmptr Ct/Mri Imag NA $84.39 0178T 64 Lead Ecg W/I&R M M 0179T 64 Lead Ecg W/Tracing M M 0180T 64 Lead Ecg W/I&R Only M M 0190T Place Intraoc Radiation Src M M 0191T Insert Ant Segment Drain Int $379.27 $379.27 0195T Prescrl Fuse W/O Instr L5/S1 NA M 0196T Prescrl Fuse W/O Instr L4/L5 NA M 0198T Ocular Blood Flow Measure M M 0200T Perq Sacral Augmt Unilat Inj $987.92 $243.46 0201T Perq Sacral Augmt Bilat Inj $553.69 $120.64 0202T Post Vert Arthrplst 1 Lumbar $4,128.40 $288.24 0205T Inirs Each Vessel Add-On NA $63.19 0206T Cptr Dbs Alys Car Elec Dta $14.66 $14.66 0207T Clear Eyelid Gland W/Heat $136.47 $68.53 0208T Audiometry Air Only $17.43 NA 0209T Audiometry Air & Bone $20.80 NA 0210T Speech Audiometry Threshold $13.07 NA 0211T Speech Audiom Thresh & Recog $20.80 NA 0212T Compre Audiometry Evaluation $21.00 $18.42 0213T Njx Paravert W/Us Cer/Thor $107.57 $60.82 0214T Njx Paravert W/Us Cer/Thor $53.09 $34.47 0215T Njx Paravert W/Us Cer/Thor $53.29 $34.87 0216T Njx Paravert W/Us Lumb/Sac $97.66 $52.10 0217T Njx Paravert W/Us Lumb/Sac $48.93 $29.72 0218T Njx Paravert W/Us Lumb/Sac $49.13 $30.11 0219T Plmt Post Facet Implt Cerv $238.91 $129.56 0220T Plmt Post Facet Implt Thor $238.91 $129.56 0221T Plmt Post Facet Implt Lumb $236.14 $127.77 0222T Plmt Post Facet Implt Addl -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

CDER List of Licensed Biological Products With
Center for Drug Evaluation and Research List of Licensed Biological Products with (1) Reference Product Exclusivity and (2) Biosimilarity or Interchangeability Evaluations to Date DATE OF FIRST REFERENCE PRODUCT DATE OF LICENSURE LICENSURE EXCLUSIVITY EXPIRY DATE INTERCHANGEABLE (I)/ BLA STN PRODUCT (PROPER) NAME PROPRIETARY NAME (mo/day/yr) (mo/day/yr) (mo/day/yr) BIOSIMILAR (B) WITHDRAWN 125118 abatacept Orencia 12/23/05 NA NA 103575 abciximab ReoPro 12/22/94 NA NA Yes 125274 abobotulinumtoxinA Dysport 04/29/09 125057 adalimumab Humira 12/31/02 NA NA 761071 adalimumab-adaz Hyrimoz 10/30/18 B 761058 adalimumab-adbm Cyltezo 08/25/17 B 761118 adalimumab-afzb Abrilada 11/15/19 B 761024 adalimumab-atto Amjevita 09/23/16 B 761059 adalimumab-bwwd Hadlima 07/23/19 B 125427 ado-trastuzumab emtansine Kadcyla 02/22/13 125387 aflibercept Eylea 11/18/11 103979 agalsidase beta Fabrazyme 04/24/03 NA NA 125431 albiglutide Tanzeum 04/15/14 017835 albumin chromated CR-51 serum Chromalbin 02/23/76 103293 aldesleukin Proleukin 05/05/92 NA NA 103948 alemtuzumab Campath, Lemtrada 05/07/01 NA NA 125141 alglucosidase alfa Myozyme 04/28/06 NA NA 125291 alglucosidase alfa Lumizyme 05/24/10 125559 alirocumab Praluent 07/24/15 103172 alteplase, cathflo activase Activase 11/13/87 NA NA 103950 anakinra Kineret 11/14/01 NA NA 020304 aprotinin Trasylol 12/29/93 125513 asfotase alfa Strensiq 10/23/15 101063 asparaginase Elspar 01/10/78 NA NA 125359 asparaginase erwinia chrysanthemi Erwinaze 11/18/11 761034 atezolizumab Tecentriq 05/18/16 761049 avelumab Bavencio 03/23/17 -

Michigan Department of Health and Human Services Practitioner and Medical Clinic Fee Schedule July - 2015 Revised: 09/30/2015 9:58:24 AM
Michigan Department of Health and Human Services Practitioner and Medical Clinic Fee Schedule July - 2015 Revised: 09/30/2015 9:58:24 AM Code Short Description Modifier Age Range Non Fac Fee Fac Fee Effective Date** 0054T Bone Srgry Cmptr Fluor Image NA $78.63 0055T Bone Srgry Cmptr Ct/Mri Imag NA $78.63 0099T Implant Corneal Ring M M 0178T 64 Lead Ecg W/I&R M M 0179T 64 Lead Ecg W/Tracing M M 0180T 64 Lead Ecg W/I&R Only M M 0182T Hdr Elect Brachytherapy M NA 0182T Hdr Elect Brachytherapy 26 M M 0182T Hdr Elect Brachytherapy TC M NA 0190T Place Intraoc Radiation Src M M 0191T Insert Ant Segment Drain Int M M 0195T Prescrl Fuse W/O Instr L5/S1 M M 0196T Prescrl Fuse W/O Instr L4/L5 M M 0198T Ocular Blood Flow Measure M M 0200T Perq Sacral Augmt Unilat Inj NA $322.66 0201T Perq Sacral Augmt Bilat Inj $484.00 $484.00 0202T Post Vert Arthrplst 1 Lumbar NA $854.70 0205T Inirs Each Vessel Add-On NA $63.19 0206T Cptr Dbs Alys Car Elec Dta $10.89 $10.89 0207T Clear Eyelid Gland W/Heat $136.47 $68.53 0208T Audiometry Air Only $17.23 NA 0209T Audiometry Air & Bone $20.60 NA 0210T Speech Audiometry Threshold $12.88 NA 0211T Speech Audiom Thresh & Recog $20.80 NA 0212T Compre Audiometry Evaluation $20.80 $18.03 0213T Njx Paravert W/Us Cer/Thor $107.17 $61.01 0214T Njx Paravert W/Us Cer/Thor $52.69 $34.27 0215T Njx Paravert W/Us Cer/Thor $53.09 $34.67 0216T Njx Paravert W/Us Lumb/Sac $96.67 $51.70 0217T Njx Paravert W/Us Lumb/Sac $48.73 $29.52 0218T Njx Paravert W/Us Lumb/Sac $48.93 $29.91 0219T Plmt Post Facet Implt Cerv NA $410.61 0220T Plmt Post -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -
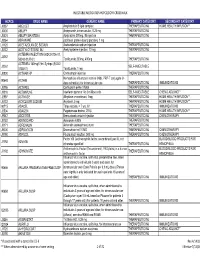
Injectable Medication Hcpcs/Dofr Crosswalk
INJECTABLE MEDICATION HCPCS/DOFR CROSSWALK HCPCS DRUG NAME GENERIC NAME PRIMARY CATEGORY SECONDARY CATEGORY J0287 ABELCET Amphotericin B lipid complex THERAPEUTIC INJ HOME HEALTH/INFUSION** J0400 ABILIFY Aripiprazole, intramuscular, 0.25 mg THERAPEUTIC INJ J0401 ABILIFY MAINTENA Apriprazole 300mg, IM injection THERAPEUTIC INJ J9264 ABRAXANE paclitaxel protein-bound particles, 1 mg J1120 ACETAZOLAMIDE SODIUM Acetazolamide sodium injection THERAPEUTIC INJ J0132 ACETYLCYSTEINE INJ Acetylcysteine injection, 10 mg THERAPEUTIC INJ ACTEMRA INJECTION (50242-0136-01, J3262 50242-0137-01) Tocilizumab 200mg, 400mg THERAPEUTIC INJ ACTEMRA 162mg/0.9ml Syringe (50242- J3262 SELF-INJECTABLE 0138-01) Tocilizumab, 1 mg J0800 ACTHAR HP Corticotropin injection THERAPEUTIC INJ Hemophilus influenza b vaccine (Hib), PRP-T conjugate (4- 90648 ACTHIB dose schedule), for intramuscular use THERAPEUTIC INJ IMMUNIZATIONS J0795 ACTHREL Corticorelin ovine triflutal THERAPEUTIC INJ J9216 ACTIMMUNE Interferon gamma 1-b 3 miillion units SELF-INJECTABLE CHEMO ADJUNCT* J2997 ACTIVASE Alteplase recombinant, 1mg THERAPEUTIC INJ HOME HEALTH/INFUSION** J0133 ACYCLOVIR SODIUM Acyclovir, 5 mg THERAPEUTIC INJ HOME HEALTH/INFUSION** 90715 ADACEL Tdap vaccine, > 7 yrs, IM THERAPEUTIC INJ IMMUNIZATIONS J2504 ADAGEN Pegademase bovine, 25 IU THERAPEUTIC INJ HOME HEALTH/INFUSION** J9042 ADCETRIS Brentuximab vedotin Injection THERAPEUTIC INJ CHEMOTHERAPY J0153 ADENOCARD Adenosine 6 MG THERAPEUTIC INJ J0171 ADRENALIN Adrenalin (epinephrine) inject THERAPEUTIC INJ J9000 ADRIAMYCIN Doxorubicin