Stevia Is a Source for Alternative Sweeteners
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tree of Life Marula Oil in Africa
HerbalGram 79 • August – October 2008 HerbalGram 79 • August Herbs and Thyroid Disease • Rosehips for Osteoarthritis • Pelargonium for Bronchitis • Herbs of the Painted Desert The Journal of the American Botanical Council Number 79 | August – October 2008 Herbs and Thyroid Disease • Rosehips for Osteoarthritis • Pelargonium for Bronchitis • Herbs of the Painted Desert • Herbs of the Painted Bronchitis for Osteoarthritis Disease • Rosehips for • Pelargonium Thyroid Herbs and www.herbalgram.org www.herbalgram.org US/CAN $6.95 Tree of Life Marula Oil in Africa www.herbalgram.org Herb Pharm’s Botanical Education Garden PRESERVING THE FULL-SPECTRUM OF NATURE'S CHEMISTRY The Art & Science of Herbal Extraction At Herb Pharm we continue to revere and follow the centuries-old, time- proven wisdom of traditional herbal medicine, but we integrate that wisdom with the herbal sciences and technology of the 21st Century. We produce our herbal extracts in our new, FDA-audited, GMP- compliant herb processing facility which is located just two miles from our certified-organic herb farm. This assures prompt delivery of freshly-harvested herbs directly from the fields, or recently HPLC chromatograph showing dried herbs directly from the farm’s drying loft. Here we also biochemical consistency of 6 receive other organic and wildcrafted herbs from various parts of batches of St. John’s Wort extracts the USA and world. In producing our herbal extracts we use precision scientific instru- ments to analyze each herb’s many chemical compounds. However, You’ll find Herb Pharm we do not focus entirely on the herb’s so-called “active compound(s)” at fine natural products and, instead, treat each herb and its chemical compounds as an integrated whole. -

Sweeteners: Innovations to Meet Consumer Preferences the Latest in Natural Sweetening Solutions
Product Development Guide Vol. 1, No. 4 July 2020 foodbeverageinsider.com US$20.75 Sweeteners: Innovations to meet consumer preferences The latest in natural sweetening solutions PAID CONTENT Sweeteners: Innovations to meet consumer preferences The latest in natural sweetening solutions Copyright © 2020 Informa Markets All rights reserved. The publisher reserves the right to accept or reject any advertising or editorial material. Advertisers, and/or their agents, assume the responsibility for all content of published advertisements and assume responsibility for any claims against the publisher based on the advertisement. Editorial contributors assume responsibility for their published works and assume responsibility for any claims against the publisher based on the published work. Editorial content may not necessarily reflect the views of the publisher. Materials contained on this site may not be reproduced, modified, distributed, republished or hosted (either directly or by linking) without our prior written permission. You may not alter or remove any trademark, copyright or other notice from copies of content. You may, however, download material from the site (one machine readable copy and one print copy per page) for your personal, noncommercial use only. We reserve all rights in and title to all material downloaded. All items submitted to Food & Beverage Insider become the sole property of Informa Markets. 2 Sweeteners: Innovations to meet consumer preferences July 2020 There’s nothing SWEETER than this. See what’s new with SupplySide West & Food ingredients North America 2020. OCTOBER 27-30, 2020 Expo Hall October 29 & 30 Mandalay Bay, Las Vegas, NV LEARN MORE supplysidewest.com Sweeteners Sweeteners: Innovations to meet consumer preferences The latest in natural sweetening solutions emember when Americans thought fat made us fat and sugar was maligned mainly for R rotting teeth? If we only knew 20 years ago what we know now—that dietary fat can be healthy while excess added sugars are a primary cause of weight gain—perhaps more than 70% of U.S. -

Popular Sweeteners and Their Health Effects Based Upon Valid Scientific Data
Popular Sweeteners and Their Health Effects Interactive Qualifying Project Report Submitted to the Faculty of the WORCESTER POLYTECHNIC INSTITUTE in partial fulfillment of the requirements for the Degree of Bachelor of Science By __________________________________ Ivan Lebedev __________________________________ Jayyoung Park __________________________________ Ross Yaylaian Date: Approved: __________________________________ Professor Satya Shivkumar Abstract Perceived health risks of artificial sweeteners are a controversial topic often supported solely by anecdotal evidence and distorted media hype. The aim of this study was to examine popular sweeteners and their health effects based upon valid scientific data. Information was gathered through a sweetener taste panel, interviews with doctors, and an on-line survey. The survey revealed the public’s lack of appreciation for sweeteners. It was observed that artificial sweeteners can serve as a low-risk alternative to natural sweeteners. I Table of Contents Abstract .............................................................................................................................................. I Table of Contents ............................................................................................................................... II List of Figures ................................................................................................................................... IV List of Tables ................................................................................................................................... -

Cargill Incorporated by Segment
BASE PROSPECTUS May 23, 2012 Cargill, Incorporated (incorporated with limited liability in the State of Delaware, United States of America) U.S.$6,000,000,000 Euro Medium Term Note Program On December 16, 1996, each of Cargill, Incorporated (‘‘Cargill, Inc.’’, ‘‘Cargill’’, the ‘‘Company’’ or the ‘‘Issuer’’), Cargill Global Funding PLC (‘‘Cargill Global’’) and Cargill Asia Pacific Treasury Ltd (‘‘Cargill Asia Pacific’’) entered into a U.S.$1,000,000,000 Euro Medium Term Note Program (the ‘‘Program’’) and issued an offering circular on that date describing the Program. The Program has been updated from time to time and several offering circulars have been issued in connection therewith. This base prospectus (the ‘‘Base Prospectus’’) further updates the Program, supersedes all previous offering circulars and/or base prospectuses and is valid for a period of 12 months as from the date hereof. Any Notes (as defined below) issued under the Program on or after the date of this Base Prospectus are issued subject to the provisions herein. This does not affect any Notes already issued. Under the Program, Cargill, Inc., may from time to time issue notes in bearer form (‘‘Bearer Notes’’) or registered form (‘‘Registered Notes’’ and, together with Bearer Notes, the ‘‘Notes’’) denominated in any currency (including euro) as agreed between Cargill, Inc. and the relevant Dealer (as defined below). Cargill, Inc. will not issue Bearer Notes unless it and any Dealer participating in the issue of such Bearer Notes has received a written opinion of independent legal counsel of recognized standing stating that payments on such Bearer Notes will not be subject to U.S. -

Pdf/ Pubjood the Primary Application of Anthocyanoside-Enriched Bilberry Food.-Contentslot- 5180- Itemlist- 23399- File
ORIGINA 1 2 IN THE UNITED STATES OF AMERICA 3 BEFORE THE FEDERAL TRADE COMMISSION OFFICE OF ADMINISTRATIVE LAW JUDGES 4 5 In the Matter of ) Docket No.: 9329 6 DANIEL CHAPTER ONE, ) ) 7 a corporation, and ) PUBLIC DOCUMENT ) 8 JAMES FEIJO, ) individually, and as an offcer of ) 9 Daniel Chapter One ) 10 ) ) 11 12 RESPONDENTS' STIPULATED MOTION TO INCLUDE EXHIBIT IN HEARING RECORD 13 14 On May 5, 2009, Complaint Counsel and Respondents' Counsel agreed - pending 15 approval by the hearing officer - that the attached THE JOURNAL OF THE AMERICAN BOTANICAL 16 COUNCIL, "HerbaIGram," No. 81 (Feb-Apr 2009) constitutes the correct and complete Exhibit 1 17 to Exhibit R18 (Deposition Transcript of James A. Duke), which was provided to the reporter at 18 the deposition but may not have been included in the final hearing record. 19 Respectfully submitted, 20 Dated: May~, 2009 21 22 ~;: 71 lJ Leonard L. Gordon, Esq. J mes S. Turner, Esq. 23 Theodore Zang, Jr., Esq. Michael McCormack, Esq Carole A. Paynter, Esq. 24 Betsy E. Lehrfeld, Esq. David W. Dulabon, Esq. Chrstopher B. Turner, Esq. 25 Elizabeth N ach, Esq. Swankin & Turner Wiliam H. Efron, Esq. 1400 16th Street, NW, Suite 101 26 Federal Trade Commission - Northeast Region Washington, DC 20036 27 One Bowling Green, Suite 318 New York, NY 10004 28 Complaint Counsel Counsel for Respondents 1 2 (PROPOSED) ORDER 3 The parties having agreed that Exhibit 1 to Exhibit R18 consists of THE JOURNAL OF THE 4 AMERICAN BOTANICAL COUNCIL, "HerbaIGram," No. 81 (Feb-Apr 2009), 5 6 IT is ORDERED that 7 To the extent it is necessary to change the hearing record such that Exhibit 1 to Exhibit 8 R18 shall consist of THE JOURNAL OF THE AMERICAN BOTANICAL COUNCIL, "HerbaIGram," No. -

Best of Sustainable Supply 2014
ETHICAL | ENVIRONMENTAL | ECONOMIC Welcome to McDonald’s 2014 Best of Sustainable Supply. SUSTAINABLE SUPPLY STEERING COMMITTEE This year, we are delighted to honor 36 suppliers and 51 ASIA-PACIFIC, MIDDLE EAST, AfRICA: Brian Kramer projects that represent real innovation toward a more EUROPE: Keith Kenny, Jacqui Macalister sustainable supply chain. Innovation is key to our CSR and sustainability journey, and McDonald’s suppliers LATIN AMERICA: Leonardo Lima, Cristina Gomez-Garcia have an impressive track record of innovating for what NORTH AMERICA: Susan Forsell, Jeff Fitzpatrick-Stilwell, we call sustainability’s three Es: ethics, environment, and Jessica Droste Yagan economics. GLOBAL: Townsend Bailey, Michele Banik-Rake, Bruce Feinberg, Gary Johnson, Rona Starr All of their efforts are impressive, and the Best of Sustainable Supply helps us to identify the very best Fifty-one case studies highlighting EXECUTIVE & EXTERNAL SELECTION PANEL practices and innovations happening every day in our sustainable supply best practices BSR: Christine Bader supply chain, and to share that knowledge with other from around the world CONSERVATION INTERNATIONAL: Bambi Semroc suppliers. This year, we received 585 submissions, almost FARM ANIMAL INITIATIVE: Roland Bonney 40 percent more than last time. I’m delighted with the FORUM FOR THE FUTURE: Helen Clarkson increase, not just because we have even more outstanding TABLE OF CONTENTS initiatives taking place in our supply chain, but because GLOBAL ROUNDTABLE FOR SUSTAINABLE BEEF: Ruaraidh Petre of what it says about McDonald’s suppliers. Every year, Climate Change and Energy 4 GREENBIZ GROUP: John Davies our suppliers focus even more attention on sustainability, McDONALD’s CORPORATION: Francesca DeBiase, Bob Langert Water applying innovation to make a real difference for the 9 THE SUSTAINABILITY CONSORTIUM: Kara Hurst people, communities, animals and environment that touch Waste SUSTAINABLE FOOD LAB: Hal Hamilton our supply chain. -

Stevia and Saccharin Preferences in Rats and Mice
Chem. Senses 35: 433–443, 2010 doi:10.1093/chemse/bjq033 Advance Access publication April 22, 2010 Stevia and Saccharin Preferences in Rats and Mice Anthony Sclafani1,2, Mahsa Bahrani1, Steven Zukerman1,2 and Karen Ackroff1,2 1Department of Psychology, Brooklyn College, 2900 Bedford Avenue, Brooklyn, NY 11210 USA and 2The Graduate Center, City University of New York, 365 Fifth Avenue, New York, NY 10016 USA Correspondence to be sent to: Anthony Sclafani, Department of Psychology, Brooklyn College, Brooklyn, NY 11210, USA. e-mail: [email protected] Accepted March 23, 2010 Abstract Use of natural noncaloric sweeteners in commercial foods and beverages has expanded recently to include compounds from the plant Stevia rebaudiana. Little is known about the responses of rodents, the animal models for many studies of taste systems and food intake, to stevia sweeteners. In the present experiments, preferences of female Sprague–Dawley rats and C57BL/6J mice for different stevia products were compared with those for the artificial sweetener saccharin. The stevia component rebaudioside A has the most sweetness and least off-tastes to human raters. In ascending concentration tests (48-h sweetener vs. water), rats and mice preferred a high-rebaudioside, low-stevioside extract as strongly as saccharin, but the extract stimulated less overdrinking and was much less preferred to saccharin in direct choice tests. Relative to the extract, mice drank more pure rebaudioside A and showed stronger preferences but still less than those for saccharin. Mice also preferred a commercial mixture of rebaudioside A and erythritol (Truvia). Similar tests of sweet receptor T1R3 knockout mice and brief- access licking tests with normal mice suggested that the preferences were based on sweet taste rather than post-oral effects. -

GRAS Notice 768 for Stevia Leaf Extract
GRAS Notice (GRN) No. 768 https://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/default.htm MAR 1 9 2018 March 14, 2018 OFFICE OF Dr. Paulette Gaynor FOOD ADDillVE SAFETY Office of Food Additive Safety (HFS-200) Center for Food Safety and Applied Nutrition Food and Drug Administration 5001 Campus Drive College Park, MD 20740-3835 Dear Dr. Gaynor RE: GRAS Exemption Claim for Stevia Leaf Extracts In accordance with proposed 21 CFR §170.36 [Notice of a claim for exemption based on a Generally Recognized as Safe (GRAS) determination] published in the Federal Register (62 FR 18938 (17 April 1997)), I am submitting one hard copy and one electronic copy (on CD), as the notifier [Cargill, Incorporated, 15407 McGinty Road West, M.S. 163, Wayzata, Minnesota, 55391], a Notice of the determination, on the basis of scientific procedures, that stevia leaf extracts, as defined in the enclosed documents and manufactured according to current Good Manufacturing Practices, is GRAS under specific conditions of use as an ingredient in food and beverages, and therefore, is exempt from the premarket approval requirements of the Federal Food, Drug, And Cosmetic Act. Information setting forth the basis for the GRAS determination, which includes detailed information on the notified substance and a summary of the basis for the GRAS determination, as well as a consensus opinion of an independent panel of experts in support of the safety of stevia leaf extracts under the intended conditions of use, also are enclosed for review by the agency. The enclosed electronic files for the Notice entitled, "GRAS Notice for Stevia Leaf Extracts" were scanned for viruses prior to submission and is thus certified as being virus-free using Symantec Endpoint Protection 12.1.5. -

Developing a Sustainable Supply Chain
ECM Truvia:Layout 1 29/11/10 10:01 Page 1 Corporate focus: Developing a sustainable supply chain 3 The sweetener story 6 Sustainable stevia from Argentina 10 A market niche for Truvia? 11 Environmentalist perspective Corporate focus supported by ECM Truvia:Layout 1 29/11/10 10:01 Page 2 2 Supply chain focus Ethical Corporation • December 2010-January 2011 Introduction Comment Strong environmental, An intriguing supply economic and social chain model standards By Zanna McFerson By Toby Webb argill has been planning a stevia business for the past seven ustainable supply chains are among the biggest responsible Cyears with a vision to build a sustainable enterprise that Sbusiness challenges. And this is not least when combined with supports growers and delivers a natural zero-calorie sweetener to the on-going debate about what we eat. We want to eat healthily consumers. – and feed healthy foods to our children – and also want to be sure In building a commercial supply chain from scratch, Cargill can that food products are developed and produced in a responsible leverage decades of experience in many mature and developing and sustainable manner. agricultural businesses within the company. For the Truvia™ In establishing the supply chain for its Truvia sweetener brand sweetener business, our team embraced the opportunity to – made from the stevia plant, a native of South America – Cargill create a responsible and sustainable supply chain that delivers has got a lot of the basics right. The company has identified on its future promises. The Truvia™ enterprise offers consumers supplier partners who are not only able to guarantee the right the taste of natural “sweet” without the calories while making quality and quantity of the raw ingredients, but also have the a responsible decision for the environment, the growers and experience to produce the product in balance with their land. -

How Safe Is Flying?
OFFBEAT NZ TONIGHT SPORT MUSIC Ring down Key’s wage McCullum just The Boss the drain freeze the first is back PAGE 2 PAGE 4 PAGE 11 PAGE 14 TGIFEDITION.TV Auckland Hamilton Wellington Christchurch Queenstown Dunedin Sat: 25°/16° Sun: 25°/15° Sat: 28°/11° Sun: 27°/13° Sat: 22°/16° Sun: 20°/16° Sat: 27°/16° Sun: 26°/14° Sat: 26°/15° Sun: 24°/13° Sat: 25°/17° Sun: 24°/14° THERE’S ONE EASY WAY TO GET THIS SUBSCRIBE TODAY, ONLY $3 PER MONTH DELIVERED TO YOUR INBOX EVERY WEEK... www.tgifedition.com EDITION ISSN 1172-4153 | Volume 2 | Issue 22 | | 23 January 2009 on the How safe INSIDE is flying? HILLARY’S MISSION Air NZ crash may be The Middle East beckons Page 5 linked to other mishaps By Ian Wishart A string of inflight failures on Airbus jetliners are prompting speculation about the safety of the planes. This afternoon, Airbus announced new safety check procedures for its A320 craft as a result of PROFITS OF preliminary investigations into the Air New Zealand crash in the Mediterranean. That warning comes only DOOM Crossfire Curson’s analysis 24 hours after revelations that the A320 that ditched miraculously in New York’s Hudson river last Friday Motorist killed during shootout Page 7 had suffered an inflight failure just two days earlier. In Air New Zealand’s case, initial data from the black box recorders has confirmed the jetliner suf- AUCKLAND, JAN 23 – An innocent driver was shot events ended as police vehicles surrounded a stopped SLUMDOG NOD fered a massive power surge just as it was coming in and killed in Auckland today and another wounded after white van and a light truck. -
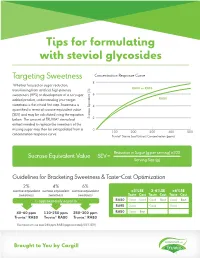
Tips for Formulating with Steviol Glycosides
Tips for formulating with steviol glycosides Targeting Sweetness Concentration Response Curve 8 Whether focused on sugar reduction, RA80 or RA95 transitioning from artificial high potency sweeteners (HPS) or development of a no-sugar 6 added product, understanding your target RA50 sweetness is the critical first step. Sweetness is 4 quantified in terms of sucrose equivalent value (SEV) and may be calculated using the equation below. The amount of TRUVIA® stevia leaf (%) Sucrose Equivalent 2 extract needed to replace the sweetness of the | | | | | missing sugar may then be extrapolated from a 0 100 200 300 400 500 concentration-response curve. Truvía® Stevia Leaf Extract Concentration (ppm) Reduction in Sugar (g per serving) x100 Sucrose Equivalent Value SEV = Serving Size (g) Guidelines for Bracketing Sweetness & Taste-Cost Optimization 2% 4% 6% sucrose equivalent sucrose equivalent sucrose equivalent <3%SE 3-6%SE >6%SE sweetness sweetness sweetness Taste Cost Taste Cost Taste Cost is approximately equal to RA80 Good Good Good Best Good Best RA95 Good Good Good 40-60 ppm 110-150 ppm 250-300 ppm RA50 Good Best Truvia® RA80 Truvia® RA80 Truvia® RA80 EU maximum use level 240 ppm RA80 (approximately 5.5% SEV) Brought to You by Cargill Optimizing Sweetness It is well established that HPS reach a maximum sweetness plateau. At higher concentrations, increasing use level yields little incremental sweetness and introduces undesirable off-tastes. In highly sweetened applications (ex. greater than 6-8% sucrose equivalent sweetness), it is often beneficial to blend with another sweetener, like Zerose® erythritol, sugar or other nutritive sweeteners. Zerose® erythritol may be used up to 3.5% in typical beverage applications. -

Settlement Terms, As Approved by Cargill’S Counsel and Class
CASE 0:13-cv-02563-RHK-JJG Document 7 Filed 09/19/13 Page 1 of 73 UNITED STATES DISTRICT COURT FOR THE DISTRICT OF MINNESOTA Molly Martin and Lauren Barry, on behalf of themselves and all others similarly situated, Civil Action No. Plaintiff, v. CLASS SETTLEMENT Cargill, Incorporated, AGREEMENT Defendant. CASE 0:13-cv-02563-RHK-JJG Document 7 Filed 09/19/13 Page 2 of 73 TABLE OF CONTENTS I. RECITALS ........................................................................................................ 4 II. DEFINITIONS ............................................................................................... 10 III. CERTIFICATION OF THE SETTLEMENT CLASS AND PRELIMINARY APPROVAL ..................................................................... 21 IV. SETTLEMENT CONSIDERATION AND BENEFITS .......................... 22 4.1 Administration and Settlement Funds .............................................. 23 4.2 Eligibility and Process for Obtaining a Cash or Voucher Payment ................................................................................................................ 27 4.3 Monetary Relief to Settlement Class Members: Payments of Cash Refunds or Eligible Product Vouchers. ............................................. 32 4.4 Monetary Relief for Settlement Class. ............................................... 33 4.5 Distribution to Authorized Settlement Class Members. ................ 38 4.6 Insufficient or Excess Funds in the Settlement or Administration Funds. ...................................................................................................