Insects Associated with Stevia Rebaudiana Bertoni (Bertoni), and Toxicity of Compounds from S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tree of Life Marula Oil in Africa
HerbalGram 79 • August – October 2008 HerbalGram 79 • August Herbs and Thyroid Disease • Rosehips for Osteoarthritis • Pelargonium for Bronchitis • Herbs of the Painted Desert The Journal of the American Botanical Council Number 79 | August – October 2008 Herbs and Thyroid Disease • Rosehips for Osteoarthritis • Pelargonium for Bronchitis • Herbs of the Painted Desert • Herbs of the Painted Bronchitis for Osteoarthritis Disease • Rosehips for • Pelargonium Thyroid Herbs and www.herbalgram.org www.herbalgram.org US/CAN $6.95 Tree of Life Marula Oil in Africa www.herbalgram.org Herb Pharm’s Botanical Education Garden PRESERVING THE FULL-SPECTRUM OF NATURE'S CHEMISTRY The Art & Science of Herbal Extraction At Herb Pharm we continue to revere and follow the centuries-old, time- proven wisdom of traditional herbal medicine, but we integrate that wisdom with the herbal sciences and technology of the 21st Century. We produce our herbal extracts in our new, FDA-audited, GMP- compliant herb processing facility which is located just two miles from our certified-organic herb farm. This assures prompt delivery of freshly-harvested herbs directly from the fields, or recently HPLC chromatograph showing dried herbs directly from the farm’s drying loft. Here we also biochemical consistency of 6 receive other organic and wildcrafted herbs from various parts of batches of St. John’s Wort extracts the USA and world. In producing our herbal extracts we use precision scientific instru- ments to analyze each herb’s many chemical compounds. However, You’ll find Herb Pharm we do not focus entirely on the herb’s so-called “active compound(s)” at fine natural products and, instead, treat each herb and its chemical compounds as an integrated whole. -

Sweeteners: Innovations to Meet Consumer Preferences the Latest in Natural Sweetening Solutions
Product Development Guide Vol. 1, No. 4 July 2020 foodbeverageinsider.com US$20.75 Sweeteners: Innovations to meet consumer preferences The latest in natural sweetening solutions PAID CONTENT Sweeteners: Innovations to meet consumer preferences The latest in natural sweetening solutions Copyright © 2020 Informa Markets All rights reserved. The publisher reserves the right to accept or reject any advertising or editorial material. Advertisers, and/or their agents, assume the responsibility for all content of published advertisements and assume responsibility for any claims against the publisher based on the advertisement. Editorial contributors assume responsibility for their published works and assume responsibility for any claims against the publisher based on the published work. Editorial content may not necessarily reflect the views of the publisher. Materials contained on this site may not be reproduced, modified, distributed, republished or hosted (either directly or by linking) without our prior written permission. You may not alter or remove any trademark, copyright or other notice from copies of content. You may, however, download material from the site (one machine readable copy and one print copy per page) for your personal, noncommercial use only. We reserve all rights in and title to all material downloaded. All items submitted to Food & Beverage Insider become the sole property of Informa Markets. 2 Sweeteners: Innovations to meet consumer preferences July 2020 There’s nothing SWEETER than this. See what’s new with SupplySide West & Food ingredients North America 2020. OCTOBER 27-30, 2020 Expo Hall October 29 & 30 Mandalay Bay, Las Vegas, NV LEARN MORE supplysidewest.com Sweeteners Sweeteners: Innovations to meet consumer preferences The latest in natural sweetening solutions emember when Americans thought fat made us fat and sugar was maligned mainly for R rotting teeth? If we only knew 20 years ago what we know now—that dietary fat can be healthy while excess added sugars are a primary cause of weight gain—perhaps more than 70% of U.S. -

The Influence of Prairie Restoration on Hemiptera
CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Thesis Submitted to The College of Arts and Sciences of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Biology By Stephanie Kay Gunter, B.A. Dayton, Ohio August 2021 CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Name: Gunter, Stephanie Kay APPROVED BY: Chelse M. Prather, Ph.D. Faculty Advisor Associate Professor Department of Biology Ryan W. McEwan, Ph.D. Committee Member Associate Professor Department of Biology Mark G. Nielsen Ph.D. Committee Member Associate Professor Department of Biology ii © Copyright by Stephanie Kay Gunter All rights reserved 2021 iii ABSTRACT CAN THE ONE TRUE BUG BE THE ONE TRUE ANSWER? THE INFLUENCE OF PRAIRIE RESTORATION ON HEMIPTERA COMPOSITION Name: Gunter, Stephanie Kay University of Dayton Advisor: Dr. Chelse M. Prather Ohio historically hosted a patchwork of tallgrass prairies, which provided habitat for native species and prevented erosion. As these vulnerable habitats have declined in the last 200 years due to increased human land use, restorations of these ecosystems have increased, and it is important to evaluate their success. The Hemiptera (true bugs) are an abundant and varied order of insects including leafhoppers, aphids, cicadas, stink bugs, and more. They play important roles in grassland ecosystems, feeding on plant sap and providing prey to predators. Hemipteran abundance and composition can respond to grassland restorations, age of restoration, and size and isolation of habitat. -

Insects & Spiders of Kanha Tiger Reserve
Some Insects & Spiders of Kanha Tiger Reserve Some by Aniruddha Dhamorikar Insects & Spiders of Kanha Tiger Reserve Aniruddha Dhamorikar 1 2 Study of some Insect orders (Insecta) and Spiders (Arachnida: Araneae) of Kanha Tiger Reserve by The Corbett Foundation Project investigator Aniruddha Dhamorikar Expert advisors Kedar Gore Dr Amol Patwardhan Dr Ashish Tiple Declaration This report is submitted in the fulfillment of the project initiated by The Corbett Foundation under the permission received from the PCCF (Wildlife), Madhya Pradesh, Bhopal, communication code क्रम 車क/ तकनीकी-I / 386 dated January 20, 2014. Kanha Office Admin office Village Baherakhar, P.O. Nikkum 81-88, Atlanta, 8th Floor, 209, Dist Balaghat, Nariman Point, Mumbai, Madhya Pradesh 481116 Maharashtra 400021 Tel.: +91 7636290300 Tel.: +91 22 614666400 [email protected] www.corbettfoundation.org 3 Some Insects and Spiders of Kanha Tiger Reserve by Aniruddha Dhamorikar © The Corbett Foundation. 2015. All rights reserved. No part of this book may be used, reproduced, or transmitted in any form (electronic and in print) for commercial purposes. This book is meant for educational purposes only, and can be reproduced or transmitted electronically or in print with due credit to the author and the publisher. All images are © Aniruddha Dhamorikar unless otherwise mentioned. Image credits (used under Creative Commons): Amol Patwardhan: Mottled emigrant (plate 1.l) Dinesh Valke: Whirligig beetle (plate 10.h) Jeffrey W. Lotz: Kerria lacca (plate 14.o) Piotr Naskrecki, Bud bug (plate 17.e) Beatriz Moisset: Sweat bee (plate 26.h) Lindsay Condon: Mole cricket (plate 28.l) Ashish Tiple: Common hooktail (plate 29.d) Ashish Tiple: Common clubtail (plate 29.e) Aleksandr: Lacewing larva (plate 34.c) Jeff Holman: Flea (plate 35.j) Kosta Mumcuoglu: Louse (plate 35.m) Erturac: Flea (plate 35.n) Cover: Amyciaea forticeps preying on Oecophylla smargdina, with a kleptoparasitic Phorid fly sharing in the meal. -
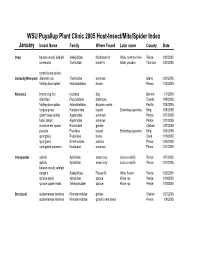
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Popular Sweeteners and Their Health Effects Based Upon Valid Scientific Data
Popular Sweeteners and Their Health Effects Interactive Qualifying Project Report Submitted to the Faculty of the WORCESTER POLYTECHNIC INSTITUTE in partial fulfillment of the requirements for the Degree of Bachelor of Science By __________________________________ Ivan Lebedev __________________________________ Jayyoung Park __________________________________ Ross Yaylaian Date: Approved: __________________________________ Professor Satya Shivkumar Abstract Perceived health risks of artificial sweeteners are a controversial topic often supported solely by anecdotal evidence and distorted media hype. The aim of this study was to examine popular sweeteners and their health effects based upon valid scientific data. Information was gathered through a sweetener taste panel, interviews with doctors, and an on-line survey. The survey revealed the public’s lack of appreciation for sweeteners. It was observed that artificial sweeteners can serve as a low-risk alternative to natural sweeteners. I Table of Contents Abstract .............................................................................................................................................. I Table of Contents ............................................................................................................................... II List of Figures ................................................................................................................................... IV List of Tables ................................................................................................................................... -

Morpho-Anatomical Study of Stevia Rebaudiana Roots Grown in Vitro and in Vivo
Revista Brasileira de Farmacognosia 27 (2017) 34–39 ww w.elsevier.com/locate/bjp Original Article Morpho-anatomical study of Stevia rebaudiana roots grown in vitro and in vivo a a a b c Rafael V. Reis , Talita P.C. Chierrito , Thaila F.O. Silva , Adriana L.M. Albiero , Luiz A. Souza , d a,b a,b,∗ José E. Gonc¸ alves , Arildo J.B. Oliveira , Regina A.C. Gonc¸ alves a Programa de Pós-graduac¸ ão em Ciências Farmacêuticas, Universidade Estadual de Maringá, Maringá, PR, Brazil b Departamento de Farmácia, Universidade Estadual de Maringá, Campus Universitário, Maringá, PR, Brazil c Departamento de Biologia, Universidade Estadual de Maringá, Campus Universitário, Maringá, PR, Brazil d Programa de Mestrado em Promoc¸ ão da Saúde, Centro Universitário de Maringá, Maringá, PR, Brazil a r a b s t r a c t t i c l e i n f o Article history: Stevia rebaudiana (Bertoni) Bertoni, Asteraceae, is used as a food additive because its leaves are a source Received 16 May 2016 of steviol glycosides. There are examples of tissue culture based on micropropagation and phytochemical Accepted 14 August 2016 production of S. rebaudiana leaves but there are few studies on adventitious root culture of S. rebaudiana. Available online 7 October 2016 More than 90% of the plants used in industry are harvested indiscriminately. In order to overcome this situation, the development of methodologies that employ biotechnology, such as root culture, provides Keywords: suitable alternatives for the sustainable use of plants. The aim of this study was to compare morpho- Stevia rebaudiana anatomical transverse sections of S. -

Nutritional and Medicinal Properties of Stevia Rebaudiana
Review Article Curr Res Diabetes Obes J Volume 13 Issue 4 - July 2020 Copyright © All rights are reserved by Fasiha Ahsan DOI: 10.19080/CRDOJ.2020.13.555867 Nutritional and Medicinal Properties of Stevia Rebaudiana Fasiha Ahsan*, Shahid Bashir and Faiz-ul-Hassan Shah University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Submission: June 25, 2020; Published: July 16, 2020 *Corresponding author: Fasiha Ahsan, PhD Scholar, University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Abstract Researches on new molecules with the least toxic effects and better potency is on its way and more attention is being given upon medicinal plants for forcing away the above problems. Medicinal plants have been recognized as potential drug candidates. Stevia, a natural sweetener with medicinal properties and also having nutritional, therapeutic and industrial importance is being used all over the world. Stevia rebaudiana leaves are usually referred to as candy, sweet and honey leaves. Diterpene glycosides are responsible for its high sweetening potential of leaves. The phytochemical properties of bioactive chemicals present in stevia leaves are involves in maintaining the physiological functions of human body. Paper also highlights the importance of nutritional aspects of dried stevia leaves, metabolism of stevia, effects of it consumption on human health and clinical studies related to stevia ingestion. Various medicinal properties of stevia leaves discussed in paper like anti-hyperglycemia, anti-oxidative, hypotensive, nephro-protective, hepato protective, antibacterial and antifungal. Basic purpose of this review to understand the medicinalKeywords: potential Stevia; Diabetes;of stevia and Phytochemicals; its acceptance Medicinal as a significant plant; Steviol;raw material Nutrition; for human Disorders diet. -

Chromosomal and Morphological Studies of Diploid and Polyploid Cytotypes of Stevia Rebaudiana (Bertoni) Bertoni (Eupatorieae, Asteraceae)
Genetics and Molecular Biology, 27, 2, 215-222 (2004) Copyright by the Brazilian Society of Genetics. Printed in Brazil www.sbg.org.br Research Article Chromosomal and morphological studies of diploid and polyploid cytotypes of Stevia rebaudiana (Bertoni) Bertoni (Eupatorieae, Asteraceae) Vanessa M. de Oliveira1, Eliana R. Forni-Martins1, Pedro M. Magalhães2 and Marcos N. Alves2 1Universidade Estadual de Campinas, Instituto de Biologia, Departamento de Botânica, Campinas, SP, Brazil. 2Universidade Estadual de Campinas, Centro Pluridisciplinar de Pesquisas Químicas, Biológicas e Agrícolas, Campinas, SP, Brazil. Abstract In this study, we examined the chromosome number and some morphological features of strains of Stevia rebaudiana. The chromosomes were analyzed during mitosis and diakinesis, and the tetrad normality and pollen viability were also assessed. In addition, stomata and pollen were measured and some plant features were studied morphometrically. All of the strains had 2n = 22, except for two, which had 2n = 33 and 2n = 44. Pairing at diakinesis wasn=11II for all of the diploid strains, whereas the triploid and tetraploid strains hadn=11III andn=11IV, respectively. Triploid and tetraploid plants had a lower tetrad normality rate than the diploids. All of the strains had inviable pollen. Thus, the higher the ploidy number, the greater the size of the pollen and the stomata, and the lower their number per unit area. The triploid strain produced the shortest plants and the lowest number of inflorescences, whereas the tetraploid strain had the largest leaves. Analysis of variance revealed highly significant differences among the strains, with a positive correlation between the level of ploidy and all of the morphological features examined. -

Insect Classification Standards 2020
RECOMMENDED INSECT CLASSIFICATION FOR UGA ENTOMOLOGY CLASSES (2020) In an effort to standardize the hexapod classification systems being taught to our students by our faculty in multiple courses across three UGA campuses, I recommend that the Entomology Department adopts the basic system presented in the following textbook: Triplehorn, C.A. and N.F. Johnson. 2005. Borror and DeLong’s Introduction to the Study of Insects. 7th ed. Thomson Brooks/Cole, Belmont CA, 864 pp. This book was chosen for a variety of reasons. It is widely used in the U.S. as the textbook for Insect Taxonomy classes, including our class at UGA. It focuses on North American taxa. The authors were cautious, presenting changes only after they have been widely accepted by the taxonomic community. Below is an annotated summary of the T&J (2005) classification. Some of the more familiar taxa above the ordinal level are given in caps. Some of the more important and familiar suborders and families are indented and listed beneath each order. Note that this is neither an exhaustive nor representative list of suborders and families. It was provided simply to clarify which taxa are impacted by some of more important classification changes. Please consult T&J (2005) for information about taxa that are not listed below. Unfortunately, T&J (2005) is now badly outdated with respect to some significant classification changes. Therefore, in the classification standard provided below, some well corroborated and broadly accepted updates have been made to their classification scheme. Feel free to contact me if you have any questions about this classification. -

Stevia Is a Subtropical Perennial That Produces Sweet Steviol Glycosides in the Leaves for Which It Also Known As ‘Cheeni Tulsi’ Or ‘Mou Tulsi’
STEVIA (Stevia rebaudiana, Asteraceae) Stevia is a subtropical perennial that produces sweet steviol glycosides in the leaves for which it also known as ‘Cheeni Tulsi’ or ‘Mou Tulsi’. Plants grown at higher latitudes actually have a higher percentage of sweet glycosides. The plant can be utilized as a source for the production of natural sweetener (food), as a source of chlorophyll, phytosterols (non-food: medicine). The sweetener can be converted into gibberellins by fermentation (Non-food — Agrochemicals), the vegetative residue can be used as animal feed and the stalks can be used as a source of cellulose (Non-food: Cellulose industry). Its medicinal uses include regulating blood sugar, preventing hypertension, treatment of skin disorders, and prevention of tooth decay. The compound obtained from stevia is considered to be the best alternate source for diabetes sufferer. The added value for this new crop can go up to a considerable extent. Market potential Market opportunity appears great. Statistics indicate that in some countries up to 30 % of their needed sugar is replaced by stevioside-like sweetness products. Soil Stevia prefers a well-drained fertile sandy loam or loam soil, high in organic matter with ample supply of water. It prefers acidic to neutral (pH 6-7) soil for better growth. It requires a consistent supply of moisture, but not waterlogged. Too much soil moisture can cause rot. Climate It is a semi-humid subtropical plant that shows higher leaf production under high light intensity and warm temperature. Day length is more critical than light intensity. Long spring and summer days favour leaf growth. -

Chromolaena Odorata Newsletter
NEWSLETTER No. 19 September, 2014 The spread of Cecidochares connexa (Tephritidae) in West Africa Iain D. Paterson1* and Felix Akpabey2 1Department of Zoology and Entomology, Rhodes University, PO Box 94, Grahamstown, 6140, South Africa 2Council for Scientific and Industrial Research (CSIR), PO Box M.32, Accra, Ghana Corresponding author: [email protected] Chromolaena odorata (L.) R.M. King & H. Rob. that substantial levels of control have been achieved and that (Asteraceae: Eupatorieae) is a shrub native to the Americas crop yield has increased by 50% due to the control of the that has become a problematic invasive in many of the weed (Day et al. 2013a,b). In Timor Leste the biological tropical and subtropical regions of the Old World (Holm et al. control agent has been less successful, possibly due to the 1977, Gautier 1992). Two distinct biotypes, that can be prolonged dry period on the island (Day et al. 2013c). separated based on morphological and genetic characters, are recognised within the introduced distribution (Paterson and Attempts to rear the fly on the SA biotype have failed Zachariades 2013). The southern African (SA) biotype is only (Zachariades et al. 1999) but the success of C. connexa in present in southern Africa while the Asian/West African (A/ South-East Asia indicates that it may be a good option for WA) biotype is present in much of tropical and subtropical control of the A/WA biotype in West Africa. A colony of the Asia as well as tropical Africa (Zachariades et al. 2013). The fly was sent to Ghana with the intention of release in that first records of the A/WA biotype being naturalised in Asia country in the 1990s but the colony failed before any releases were in India and Bangladesh in the 1870s but it was only in were made (Zachariades et al.