The Genetics of Deafness in Domestic Animals
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
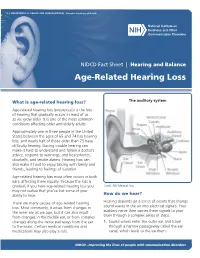
Age-Related Hearing Loss
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Age-Related Hearing Loss What is age-related hearing loss? The auditory system Age-related hearing loss (presbycusis) is the loss of hearing that gradually occurs in most of us as we grow older. It is one of the most common conditions affecting older and elderly adults. Approximately one in three people in the United States between the ages of 65 and 74 has hearing loss, and nearly half of those older than 75 have difficulty hearing. Having trouble hearing can make it hard to understand and follow a doctor’s advice, respond to warnings, and hear phones, doorbells, and smoke alarms. Hearing loss can also make it hard to enjoy talking with family and friends, leading to feelings of isolation. Age-related hearing loss most often occurs in both ears, affecting them equally. Because the loss is gradual, if you have age-related hearing loss you Credit: NIH Medical Arts may not realize that you’ve lost some of your ability to hear. How do we hear? There are many causes of age-related hearing Hearing depends on a series of events that change loss. Most commonly, it arises from changes in sound waves in the air into electrical signals. Your the inner ear as we age, but it can also result auditory nerve then carries these signals to your from changes in the middle ear, or from complex brain through a complex series of steps. changes along the nerve pathways from the ear 1. -

Novel Association of Hypertrophic Cardiomyopathy, Sensorineural Deafness, and a Mutation in Unconventional Myosin VI (MYO6)
309 LETTER TO JMG J Med Genet: first published as 10.1136/jmg.2003.011973 on 1 April 2004. Downloaded from Novel association of hypertrophic cardiomyopathy, sensorineural deafness, and a mutation in unconventional myosin VI (MYO6) S A Mohiddin, Z M Ahmed, A J Griffith, D Tripodi, T B Friedman, L Fananapazir, R J Morell ............................................................................................................................... J Med Genet 2004;41:309–314. doi: 10.1136/jmg.2003.011973 amilial hypertrophic cardiomyopathy (FHC) is typically Key points characterised by left ventricular hypertrophy, diastolic Fdysfunction, and hypercontractility, and is often asso- ciated with disabling symptoms, arrhythmias, and sudden N Familial hypertrophic cardiomyopathy (FHC) is typi- death.1 FHC shows both non-allelic and allelic genetic cally confined to a cardiac phenotype and is caused by heterogeneity, and results from any one of more than 100 mutations in genes encoding sarcomeric proteins. mutations in genes encoding sarcomeric proteins.2 Identified Occasionally FHC may be one component of a genes include those encoding b myosin heavy chain, the hereditary multisystem disorder. myosin regulatory and essential light chains, myosin bind- N Sensorineural hearing loss is genetically heteroge- ing protein C, troponin I, troponin C, a cardiac actin, and neous. Mutations in the MYO6 gene, encoding 23 titin. The FHC phenotype is characterised by hypertrophy, unconventional myosin VI, have been found to cause myocyte disarray and fibrosis, and results from the dominant non-syndromic sensorineural hearing loss—that is, negative expression of one of these (mainly missense) sensorineural hearing loss in the absence of any other mutations. The resulting sarcomeric dysfunction leads related clinical features. ultimately, through mechanisms that remain obscure, to pathological left ventricular remodelling. -

Autism Multiplex Family with 16P11.2P12.2 Microduplication Syndrome in Monozygotic Twins and Distal 16P11.2 Deletion in Their Brother
European Journal of Human Genetics (2012) 20, 540–546 & 2012 Macmillan Publishers Limited All rights reserved 1018-4813/12 www.nature.com/ejhg ARTICLE Autism multiplex family with 16p11.2p12.2 microduplication syndrome in monozygotic twins and distal 16p11.2 deletion in their brother Anne-Claude Tabet1,2,3,4, Marion Pilorge2,3,4, Richard Delorme5,6,Fre´de´rique Amsellem5,6, Jean-Marc Pinard7, Marion Leboyer6,8,9, Alain Verloes10, Brigitte Benzacken1,11,12 and Catalina Betancur*,2,3,4 The pericentromeric region of chromosome 16p is rich in segmental duplications that predispose to rearrangements through non-allelic homologous recombination. Several recurrent copy number variations have been described recently in chromosome 16p. 16p11.2 rearrangements (29.5–30.1 Mb) are associated with autism, intellectual disability (ID) and other neurodevelopmental disorders. Another recognizable but less common microdeletion syndrome in 16p11.2p12.2 (21.4 to 28.5–30.1 Mb) has been described in six individuals with ID, whereas apparently reciprocal duplications, studied by standard cytogenetic and fluorescence in situ hybridization techniques, have been reported in three patients with autism spectrum disorders. Here, we report a multiplex family with three boys affected with autism, including two monozygotic twins carrying a de novo 16p11.2p12.2 duplication of 8.95 Mb (21.28–30.23 Mb) characterized by single-nucleotide polymorphism array, encompassing both the 16p11.2 and 16p11.2p12.2 regions. The twins exhibited autism, severe ID, and dysmorphic features, including a triangular face, deep-set eyes, large and prominent nasal bridge, and tall, slender build. The eldest brother presented with autism, mild ID, early-onset obesity and normal craniofacial features, and carried a smaller, overlapping 16p11.2 microdeletion of 847 kb (28.40–29.25 Mb), inherited from his apparently healthy father. -

PRESBYCUSIS Diagnosis and Treatment
Hear the FACTS about PRESBYCUSIS Diagnosis and Treatment NORMAL HEARING What is Presbycusis? FREQUENCY (in Hertz) . A gradual reduction in hearing as we get older, typically affecting both 250 500 1000 2000 4000 8000 -10 ears equally. 0 X 10 X X Common in men and women, with men typically having greater X •X . 20 • •X • • • 30 hearing loss than women of the same age. 40 Typically a greater hearing loss for high frequency sounds than for low 50 . (in dBHL) 60 INTENSITY frequency sounds. 70 80 A treatable condition that can benefit greatly from technological 90 . advances in various amplification or hearing assistance devices, along 100 • Right Ear 110 X Left Ear with counseling on effective communication strategies. PRESBYCUSIS HEARING LOSS What causes Presbycusis? FREQUENCY (in Hertz) . Family history or hereditary factors. 250 500 1000 2000 4000 8000 -10 Changes in the inner ear blood supply related to heart disease, 0 . 10 •X diabetes, high blood pressure and smoking. 20 •X 30 •X A loss of sound sensitivity from cumulative exposure to loud sounds. 40 •X . 50 (in dBHL) 60 INTENSITY X 70 • Symptoms: X 80 • •X 90 . Frequently asking people to repeat what they say, especially in 100 Right Ear “difficult listening places”. 110 X Left Ear . Ability to hear lower-pitched men's voices easier than higher-pitched NORMAL INNER EAR PRESBYCUSIC INNER EAR women’s or children’s voices. People complaining that the TV is being played too loud. Tinnitus, also known as “head noise”, which produces buzzing or ringing sounds in the ear. Diagnosis: . Talk honestly with your Hearing Healthcare Provider about daily hearing problems. -

Clinical Genetics and the Hutterite Brethren
Clinical Genetics and the Hutterite Brethren: What have we learned in the new millenium? Or: A Micheil Innes MD FRCPC FCCMG Adapted from: Medical Genetics Grand Rounds January 2013 History and Population Hutterite Population Today >40 000 in AB, 30000 MB, ND, SD 1593-1770 1874-1879 25000 Transylvania 1256 migrated to American Prairies 20000 15000 World War I 10000 1565-1592 1770 - 1870 Migration to Canada Moravia5000 Ukraine 0 1500s 1520 1540 1550 1570 1580 1590 1610 1620 1680 1750 1760 1840 1860 1890 1900 1950 1975 1990 Tyrolean Alps Why Identify Genes in this Population? • Direct Benefits to • Benefit to Larger Patients/Families population – Non-invasive – Most of these disorders diagnostic test are not confined to this – Carrier test (*marriage population restrictions) – May allow for diagnosis – ?Prenatal testing of atypical cases – Enhanced understanding of – Expand basic science disease may facilitate and clinical knowledge management or treatment Initial Presentations May be Non-Specific Highlighting Importance of Careful Syndrome Delineation and Early Genetic Diagnosis • Hearing Loss – Autosomal recessive non-syndromic hearing loss (> 2loci) – Usher syndrome (> 2loci) – HDR syndrome • Cerebellar Ataxia – Joubert syndrome – DES syndrome – DCMA syndrome – CASS syndrome • Muscular Dystrophy/ High CK – LGMD2H – LGDM2I – AR EDMD – Myopathy with CPEO – Microcephaly with Chorea Genetic services and the Hutterites Religion/Culture • Has posed little barrier overall • Very accepting of medical care and technology • Although they believe that God plays a day to day role in guiding their lives, most couples accept genetic explanations for their children’s disorders • Some leuts and individual colonies are more conservative than others • Colony leader is clearly the Minister • Who speaks for the overall community when it comes to community wide issues? – e.g. -

Use of Genomic Tools to Discover the Cause of Champagne Dilution Coat Color in Horses and to Map the Genetic Cause of Extreme Lordosis in American Saddlebred Horses
University of Kentucky UKnowledge Theses and Dissertations--Veterinary Science Veterinary Science 2014 USE OF GENOMIC TOOLS TO DISCOVER THE CAUSE OF CHAMPAGNE DILUTION COAT COLOR IN HORSES AND TO MAP THE GENETIC CAUSE OF EXTREME LORDOSIS IN AMERICAN SADDLEBRED HORSES Deborah G. Cook University of Kentucky, [email protected] Right click to open a feedback form in a new tab to let us know how this document benefits ou.y Recommended Citation Cook, Deborah G., "USE OF GENOMIC TOOLS TO DISCOVER THE CAUSE OF CHAMPAGNE DILUTION COAT COLOR IN HORSES AND TO MAP THE GENETIC CAUSE OF EXTREME LORDOSIS IN AMERICAN SADDLEBRED HORSES" (2014). Theses and Dissertations--Veterinary Science. 15. https://uknowledge.uky.edu/gluck_etds/15 This Doctoral Dissertation is brought to you for free and open access by the Veterinary Science at UKnowledge. It has been accepted for inclusion in Theses and Dissertations--Veterinary Science by an authorized administrator of UKnowledge. For more information, please contact [email protected]. STUDENT AGREEMENT: I represent that my thesis or dissertation and abstract are my original work. Proper attribution has been given to all outside sources. I understand that I am solely responsible for obtaining any needed copyright permissions. I have obtained needed written permission statement(s) from the owner(s) of each third-party copyrighted matter to be included in my work, allowing electronic distribution (if such use is not permitted by the fair use doctrine) which will be submitted to UKnowledge as Additional File. I hereby grant to The University of Kentucky and its agents the irrevocable, non-exclusive, and royalty-free license to archive and make accessible my work in whole or in part in all forms of media, now or hereafter known. -

Identification of Horses Booklet
colours & marking booklet_10366 21/7/08 14:32 Page 1 1 Introduction The first edition of this booklet was made available to the veterinary profession in 1930. It was in the form of a report from a Sub-Committee, which had been set up by the Council of the RCVS in 1928, to prepare a system of description, colours and markings, etc. of horses for the purpose of identifying individual animals. Since that date there have been several revised editions. Amendments have been made in the light of experience and in response to the changing demands of the equine industry. The significant increase in the international movement of horses and the insistence by a growing number of organisations which hold shows, gymkhanas, events and other competitive functions that horse owners should supply proof of vaccination against equine influenza, have resulted in greater numbers of practitioners being asked to supply the relevant certification. Additionally DEFRA legislation came into force in 2004 requiring all horses to have a passport containing an accurate set of markings. This latest edition (published May 2008) has been produced by Weatherbys, in conjunction with the RCVS and BEVA, in part as a result of the increased use internationally of microchip transponders to identify horses. Nevertheless, the passport, with its recording of a horse’s colour and markings, remains the essential means of identification for Thoroughbred and Non-Thoroughbred horses and ponies under the implementation Regulation of the EC Directives (Commission Regulation (EC) No. 504/2008 of 6 June 2008 and Council Directives 90/426/EEC and 90/427 EEC. -

Anti-DFNA5 Antibody (Internal) Rabbit Anti Human Polyclonal Antibody Catalog # ALS17405
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 Anti-DFNA5 Antibody (Internal) Rabbit Anti Human Polyclonal Antibody Catalog # ALS17405 Specification Anti-DFNA5 Antibody (Internal) - Product Information Application WB, IHC-P Primary Accession O60443 Predicted Human, Mouse, Rat Host Rabbit Clonality Polyclonal Calculated MW 54555 Anti-DFNA5 Antibody (Internal) - Additional Information Gene ID 1687 Alias Symbol DFNA5 Other Names DFNA5, ICERE-1, Deafness, autosomal dominant 5, ICERE1 Target/Specificity Recognizes endogenous levels of DFNA5 protein. Reconstitution & Storage PBS, pH 7.3, 0.01% sodium azide, 30% glycerol. Store at -20°C. Aliquot to avoid freeze/thaw cycles. Precautions Anti-DFNA5 Antibody (Internal) is for research use only and not for use in diagnostic or therapeutic procedures. Anti-DFNA5 Antibody (Internal) - Protein Information Name GSDME {ECO:0000303|PubMed:28459430, ECO:0000312|HGNC:HGNC:2810} Function [Gasdermin-E]: Precursor of a pore-forming protein that converts non-inflammatory apoptosis to pyroptosis (PubMed:<a href=" Page 1/2 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 http://www.uniprot.org/citations/27281216" target="_blank">27281216</a>, PubMed:<a href="http://www.uniprot.org/ci tations/28459430" target="_blank">28459430</a>). This form constitutes the precursor of the pore- forming protein: upon cleavage, the released N-terminal moiety (Gasdermin-E, N-terminal) binds to membranes and forms pores, triggering pyroptosis (PubMed:<a hr ef="http://www.uniprot.org/citations/28459 430" target="_blank">28459430</a>). Cellular Location [Gasdermin-E, N-terminal]: Cell membrane; Multi-pass membrane protein {ECO:0000250|UniProtKB:Q5Y4Y6} Tissue Location Expressed in cochlea (PubMed:9771715). -

Special Issue Horse Genetics DIRECTOR’S Message
CENTER FOR EQUINE HEALTH SCHOOL OF VETERINARY MEDICINE • UNIVERSITY OF CALIFORNIA, DAVIS SUMMER 2020 Special Issue Horse Genetics DIRECTOR’S Message s an equine genetics researcher, I am particularly excited to share A this special issue of the Horse Report with you. Inside, you will find a roadmap to many of the currently available equine genetic tests, including the AQHA “five-panel” test, and more. The equine genome sequence was published in 2009, the result of a years- long collaborative effort by the international equine research community. This resource drastically changed how researchers approach equine genetics and accelerated the rate of discovery. Increased availability and affordability allowed the application of advanced molecular tools to equine diseases and traits. As a result, genetic tests are available in a variety of breeds. Most available tests are for simple, Mendelian diseases and traits – those caused by a single gene or locus. Complex diseases and traits likely involve more than one gene and may be influenced by environmental effects. The 2018 release of a new equine genome sequence assembly, coupled with cost reductions that make whole-genome sequencing possible for large numbers of horses, are enabling research in these areas. As an equine geneticist and veterinarian, I am especially interested in applying whole genome sequencing and advanced diagnostic tools to equine precision medicine. This highly individualized approach will focus on early detection and prevention of disease, taking into account both genetic information and environmental factors. The idea is to target individuals based on their clinical condition as well as their unique body chemistry and genetics. -

The Show of Colours
THE SHOW OF COLOURS Celebration of every horse SHOWING CLASSES FOR ALL colour – classes for all WITH EVENING PERFORMANCE SPECTACULAR Sunday 7th July 2019 CLASSES FOR ALL HORSES AND PONIES COLOURED, TWO TONE AND SOLID £13.00 Pre entry £15.00 entry on the day Fun classes £10.00 – pre entry and on the day EVENING PERFORMANCE INCLUDING COLOUR PARADE, CLASS AND COLOUR CHAMPIONSHIPS QUALIFYING SHOW FOR CHAPS (UK) OPPORTUNITY TO GAIN POINTS FOR THE HOWE GRAND FINALE AND STEP TOWARDS THE CHANCE TO WIN £500 FOR YOURSELF AND £500 FOR YOU CHOSEN CHARITY QUALIFYING SHOW FOR OUR NEW ECOSSE ELITE TROPHY FOR SCOTTISH BREEDS THE CHANCE TO WIN £100 FOR YOURSELF AND £100 FOR YOU CHOSEN CHARITY QUALIFYING SHOW FOR THE CALEDONIAN SHOWING CHAMPIONSHIPS Please be aware that “Not Before Times” are only as a guide, classes will not start before these times but may start considerably later depending on entries Pre enter on Equo Pre entries close on 3rd July 2019 THE SHOW OF COLOURS – SUNDAY 7th JULY 2019 Welcome to the schedule of the Show of Colours which run in four rings; Ring 1 - Coloured Ring – for all Skewbald and Piebald Ring 2 -Two Tone Ring – for all Roan, Palomino, Spotted and Dun/Buckskin Ring 3 - Solid Ring – for all Black, Grey, Chestnut and Bay Ring 4 - Fun Ring - for all colours Ring 5 - TGCA Scottish Regional Show - The day of showing classes for all horses and ponies of all breeds and types will culminate in an Evening Performance Spectacular. EVENING PERFORMANCE (not before 4.30pm) CONCOURS DE ELEGANCE COLOUR PARADE Free entry – open to all please take part in hand or ridden, details on next page. -

RNA Editing at Baseline and Following Endoplasmic Reticulum Stress
RNA Editing at Baseline and Following Endoplasmic Reticulum Stress By Allison Leigh Richards A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Human Genetics) in The University of Michigan 2015 Doctoral Committee: Professor Vivian G. Cheung, Chair Assistant Professor Santhi K. Ganesh Professor David Ginsburg Professor Daniel J. Klionsky Dedication To my father, mother, and Matt without whom I would never have made it ii Acknowledgements Thank you first and foremost to my dissertation mentor, Dr. Vivian Cheung. I have learned so much from you over the past several years including presentation skills such as never sighing and never saying “as you can see…” You have taught me how to think outside the box and how to create and explain my story to others. I would not be where I am today without your help and guidance. Thank you to the members of my dissertation committee (Drs. Santhi Ganesh, David Ginsburg and Daniel Klionsky) for all of your advice and support. I would also like to thank the entire Human Genetics Program, and especially JoAnn Sekiguchi and Karen Grahl, for welcoming me to the University of Michigan and making my transition so much easier. Thank you to Michael Boehnke and the Genome Science Training Program for supporting my work. A very special thank you to all of the members of the Cheung lab, past and present. Thank you to Xiaorong Wang for all of your help from the bench to advice on my career. Thank you to Zhengwei Zhu who has helped me immensely throughout my thesis even through my panic. -

Chronic Exposure of Humans to High Level Natural Background Radiation Leads to Robust Expression of Protective Stress Response Proteins S
www.nature.com/scientificreports OPEN Chronic exposure of humans to high level natural background radiation leads to robust expression of protective stress response proteins S. Nishad1,2, Pankaj Kumar Chauhan3, R. Sowdhamini3 & Anu Ghosh1,2* Understanding exposures to low doses of ionizing radiation are relevant since most environmental, diagnostic radiology and occupational exposures lie in this region. However, the molecular mechanisms that drive cellular responses at these doses, and the subsequent health outcomes, remain unclear. A local monazite-rich high level natural radiation area (HLNRA) in the state of Kerala on the south-west coast of Indian subcontinent show radiation doses extending from ≤ 1 to ≥ 45 mGy/y and thus, serve as a model resource to understand low dose mechanisms directly on healthy humans. We performed quantitative discovery proteomics based on multiplexed isobaric tags (iTRAQ) coupled with LC–MS/MS on human peripheral blood mononuclear cells from HLNRA individuals. Several proteins involved in diverse biological processes such as DNA repair, RNA processing, chromatin modifcations and cytoskeletal organization showed distinct expression in HLNRA individuals, suggestive of both recovery and adaptation to low dose radiation. In protein–protein interaction (PPI) networks, YWHAZ (14-3-3ζ) emerged as the top-most hub protein that may direct phosphorylation driven pro- survival cellular processes against radiation stress. PPI networks also identifed an integral role for the cytoskeletal protein ACTB, signaling protein PRKACA; and the molecular chaperone HSPA8. The data will allow better integration of radiation biology and epidemiology for risk assessment [Data are available via ProteomeXchange with identifer PXD022380]. Te basic principles of low linear energy transfer (LET) ionizing radiation (IR) induced efects on mammalian systems have been broadly explored and there exists comprehensive knowledge on the health efects of high doses of IR delivered at high dose rates.