"Diversity of Life Through Time"
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Entomofauna Ansfelden/Austria; Download Unter
©Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Entomofauna ZEITSCHRIFT FÜR ENTOMOLOGIE Band 28, Heft 28: 377-388 ISSN 0250-4413 Ansfelden, 30. November 2007 Phytophagous Noctuidae (Lepidoptera) of the Western Black Sea Region and their ichneumonid parasitoids Z. OKYAR & M. YURTCAN Abstract Eleven agricultural and silviculturally important species of Noctuidae and their parasitoids were determined in 33 localities from the Western Black Sea region between 2001 and 2004. The ichneumonid biological control agents Enicospilus ramidulus, Barylypa amabilis and Itoplectis alternans were obtained by rearing the host larvae. K e y w o r d s : Lepidoptera, Noctuidae, Hymenoptera, Ichneumonidae, parasitoidism, Western Black Sea Region, Turkey Zusammenfassung 11 land- und forstwirtschaftlich bedeutende Noctuidae-Arten einschließlich ihrer Parasitoide aus 33 Standorten des Gebietes des westlichen Schwarzen Meeres wurden im Zeitraum 2001 bis 2004 studiert. Ichneumonidae der Arten Enicospilus ramidulus, Barylypa amabilis and Itoplectis alternans konnten durch Aufzucht der Wirtslarven festgestellt werden. 377 ©Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Introduction The Noctuidae is the largest family of the Lepidoptera. Larvae of some species are par- ticularly harmful to agricultural and silvicultural regions worldwide. Consequently, for years intense efforts have been carried out to control them through chemical, biological, and cultural methods (LIBURD et al. 2000; HOBALLAH et al. 2004; TOPRAK & GÜRKAN 2005). In the field, noctuid control is often carried out by parasitoid wasps (CHO et al. 2006). Ichneumonids are one of the most prevalent parasitoid groups of noctuids but they also parasitize on other many Lepidoptera, Coleoptera, Hymenoptera, Diptera and Araneae (KASPARYAN 1981; FITTON et al. 1987, 1988; GAULD & BOLTON 1988; WAHL 1993; GEORGIEV & KOLAROV 1999). -

12/04 CURRICULUM VITAE Karl W
12/04 CURRICULUM VITAE Karl W. Flessa Department of Geosciences University of Arizona Tucson, Arizona 85721 (520) 621-7336 FAX (520) 621-2672 [email protected] Personal Data Birth Date: August 3, 1946 Place of Birth: Nürnberg, Germany Citizenship: U.S.A. (since 1950) Education and Degrees Ph.D., Geological Sciences, 1973. Brown University, Providence, Rhode Island A.B., Geology, 1968. Lafayette College, Easton, Pennsylvania Principal Administrative Appointments Head, Department of Ecology and Evolutionary Biology, University of Arizona, 1997-1999 Project Director, Research Training Group: Analysis of Biological Diversification, 1992-1994 Program Director, National Science Foundation, 1988-1990 Director, University of Arizona Geology Field Camp; 1983, 1985-1988 Academic Appointments Fellow, Udall Center for Studies in Public Policy, 2002-2003 Professor of Geosciences, University of Arizona, 1987-present Professor of Ecology & Evolutionary Biology, University of Arizona, 1997-present Visiting Professor, Facultad de Ciencias Marinas, Universidad Autónoma de Baja California, Ensenada, México, 1999 Research Associate, Smithsonian Institution, 1988-1991 Associate Professor, University of Arizona; 1981-1987 Humboldt Fellow, Universität Tübingen, Germany, and University of Birmingham, U.K.; 1983- 1984 Research Associate, Arizona-Sonora Desert Museum; 1978-present Assistant Professor, University of Arizona; 1977-1981 Assistant Professor, State University of New York at Stony Brook; 1972-1977 Senior Assistant, Marine Biological Laboratory; 6/72-8/72 Lecturer, Case Western Reserve University; 1/71-6/71 Research Interests Current research interests focus on 1) the environmental history and conservation biology of the Colorado River Delta, 2) the taphonomy and paleoecology of Recent and Pleistocene invertebrates in the Gulf of California, 3) the quality of the fossil record, and 4) the biogeography of Recent bivalve mollusks. -

Ewsletter Before May 15
EWSLET TER of the MICHIGAN ENTOMOLOGICAL SOCIETY 2&3 April 25, 1986 T h i r t y - sec 0 n d An nua I M e e tin 9 P I ann e d Mark O'Brien has prepared an excellent ~ session for this year's annual meeting at the ~ University of Michigan's Matthaei Botanical ~ Gardens. Our featured speaker will be Robert Matthews from the University of Georgia. The extra symposium topic planned this year focu (-----: ses on the Great Lakes Region fauna. There will be a lobby display on "Michigan Insects." Mark chose Zingerman's Deli for the catered luncheon. He says they always have a superior spread. As an added feature, , several companies have donated products which r-· -~--, we will give away as door prizes. And don't : ,----....... forget the poster contest (rules in last ,-------, news let ter). Mark has gone all out for this meeting, {' so how about joining us! There will be the usual evening collecting, and of course, --' tours of the fabulous gardens and greenhouses. Put the date on your calendar now and send in the pre-registration form from this newsletter before May 15. See you there! 32nd Annual Meeting Michigan Entomological Society June 6, 1986 co.. 'Iz. ti\i. Matthaei Botanical Gardens 1800 Dixboro Road Ann Arbor, MICH 48105 Call: Mark O'Brien at (313) 764-0471 for further information. The NEWSLETTER of the Michigan Entomological Society is published as four numbers yearly, at irregular intervals. Please send all notes, news, new insect records, research requests, notices, season summaries, membership inquiries, etc. -

Moths of Ohio Guide
MOTHS OF OHIO field guide DIVISION OF WILDLIFE This booklet is produced by the ODNR Division of Wildlife as a free publication. This booklet is not for resale. Any unauthorized INTRODUCTION reproduction is prohibited. All images within this booklet are copyrighted by the Division of Wildlife and it’s contributing artists and photographers. For additional information, please call 1-800-WILDLIFE. Text by: David J. Horn Ph.D Moths are one of the most diverse and plentiful HOW TO USE THIS GUIDE groups of insects in Ohio, and the world. An es- Scientific Name timated 160,000 species have thus far been cata- Common Name Group and Family Description: Featured Species logued worldwide, and about 13,000 species have Secondary images 1 Primary Image been found in North America north of Mexico. Secondary images 2 Occurrence We do not yet have a clear picture of the total Size: when at rest number of moth species in Ohio, as new species Visual Index Ohio Distribution are still added annually, but the number of species Current Page Description: Habitat & Host Plant is certainly over 3,000. Although not as popular Credit & Copyright as butterflies, moths are far more numerous than their better known kin. There is at least twenty Compared to many groups of animals, our knowledge of moth distribution is very times the number of species of moths in Ohio as incomplete. Many areas of the state have not been thoroughly surveyed and in some there are butterflies. counties hardly any species have been documented. Accordingly, the distribution maps in this booklet have three levels of shading: 1. -

PRISCUM the Newsletter of the Paleontological Society Volume 13, Number 2, Fall 2004
PRISCUM The Newsletter of the Paleontological Society Volume 13, Number 2, Fall 2004 Paleontological PRESIDENT’S Society Officers COLUMN: Inside... President Treasurer’s Report 2 William I. Ausich WE NEED YOU! GSA Information 2 President-Elect by William I. Ausich Reviews of PS- David Bottjer Sponsored Sessions 3 Past-President Why are you a member of The Paleontology Portal 5 Patricia H. Kelley The Paleontological Society? In PS Lecture Program 6 Secretary the not too distance past, the Books for Review 9 Roger D. K. Thomas only way to receive a copy of the Journal of Book Reviews 9 Treasurer Paleontology and Paleobiology was to pay your dues Conference Announce- and belong to the Society. I suppose one could Mark E. Patzkowsky have borrowed a copy from a friend or wander over ments 14 JP Managing Editors to the library. However, this was probably done Ann (Nancy) F. Budd with a heavy burden of guilt. Now, as we move Christopher A. Brochu into the digital age of scientific journal publishing, Jonathan Adrain one can have copies of the Journal of Paleontology and Paleobiology transmitted right to your Paleobiology Editors computer. It actually may arrive faster than the Tomasz Baumiller U.S. mail, you do not have to pay anything, and Robyn Burnham you do not even have to walk over to the library. Philip Gingerich No need for shelf space, no hassle, no dues, no Program Coordinator guilt – isn’t the Web great? The Web is great, but the Society needs dues-paying members in order Mark A. Wilson to continue to publish in paper, digitally, or both. -

EXTERNAL GENITALIC MORPHOLOGY and COPULATORY MECHANISM of CYANOTRICHA NECYRIA (FELDER) (DIOPTIDAE) Genitalic Structure Has Been
Journal of the Lepidopterists' Society 42(2). 1988, 103-115 EXTERNAL GENITALIC MORPHOLOGY AND COPULATORY MECHANISM OF CYANOTRICHA NECYRIA (FELDER) (DIOPTIDAE) JAMES S. MILLER Curatorial Fellow, Department of Entomology, American Museum of Natural History, Central Park West at 79th Street, New York, New York 10024 ABSTRACT. External genitalia of Cyanotricha necyria (Felder) exhibit characters that occur in the Notodontidae and Dioptidae. These provide further evidence that the two groups are closely related. Dissection of two C. necyria pairs in copula revealed two features unique among copulatory mechanisms described in Lepidoptera. First, only the male vesica, rather than the aedoeagus and vesica, are inserted into the female. Secondly, during copulation the female is pulled into the male abdomen, and his eighth segment applies dorsoventral pressure on the female's seventh abdominal segment. This mechanism is facilitated by a long membrane between the male eighth and ninth abdominal segments. The first trait is probably restricted to only some dioptid species, while the second may represent a synapomorphy for a larger group that would include all dioptids, and all or some notodontids. Additional key words: Noctuoidea, Notodontidae, Josiinae, functional morphology. Genitalic structure has been one of the most important sources of character information in Lepidoptera systematics. Taxonomists often use differences in genitalic morphology to separate species, and ho mologous similarities have provided characters for defining higher cat egories in Lepidoptera classification (Mehta 1933, Mutuura 1972, Dug dale 1974, Common 1975). Unfortunately, we know little concerning functional morphology of genitalia. A knowledge of function may aid in determining homology of genitalic structures, something that has proved to be extremely difficult and controversial. -
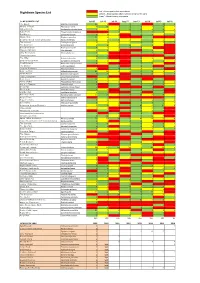
Highdown Species List 2008 and 2009 and 2010 and 2011 and 2012 and 2013 and 2014 and 2015 and 2016.Xlsx
'red' = those species that were absent Highdown Species List 'yellow' = those species who's numbers remained the same 'green' = those showing an increase CORE SURVEY LIST Jul‐09 Jul‐10 Jul‐11 Aug‐12 Jun‐13 Jul‐14 Jul‐15 Jul‐16 The Snout Hypena proboscidalis 1142322 Mother of Pearl Pleuroptya ruralis 911334242 Willow Beauty Peribatodes rhomboidaria 76368491 Ruby Tiger Phragmatobia fuliginosa 212232 Buff Ermine Spilosoma luteum 862 2913 Muslin Moth Diaphora mendica 12 6 2 1 Broad-bordered Yellow Underwing Noctua fimbriata 52621311 The Coronet Craniophora ligustri 43114725 The Sycamore Acronicta aceris 2323232 Burnished Brass Diachrysia chrysitis 632332 Chinese Character Cilix glaucata 131 21 Grey Pine Carpet Thera obeliscata 74 333 Carcina quercana 23 1 The Miller Acronicta leporine 68 642 Swallow-tailed Moth Ourapteryx sambucaria 951 6243 Small Emerald Hemistola chrysoprasaria 81 232 The Drinker Euthrix potatoria 1122 Swallow Prominent Pheosia tremula 4 34212 Rosy Footman Miltochrista miniata 4118224946 White Ermine Spilosoma lubricipeda 791 3623 True Lover's Knot Lycophotia porphyrea 1 Knot Grass Acronicta rumicis 127352 Angle Shades Phlogophora meticulosa 84114121 Nut-tree Tussock Colocasia coryli 4 4 3341 Brown-tail Euproctis chrysorrhoea 72 24 Blood-vein Timandra comae 72 1112 Slender Pug Eupithecia tenuiata 24 5 1 Brimstone Moth Opisthograptis luteolata 11 19 2 7 12 6 6 4 Purple Thorn Selenia tetralunaria 1 2 Peppered Moth Biston betularia 75126253 Buff Arches Habrosyne pyritoides 532 4464 Setaceous Hebrew Character Xestia c‐nigrum -

Similarity of Mammalian Body Size Across the Taxonomic Hierarchy and Across Space and Time Felisa A
University of New Mexico UNM Digital Repository Biology Faculty & Staff ubP lications Scholarly Communication - Departments 5-1-2004 Similarity of Mammalian Body Size across the Taxonomic Hierarchy and across Space and Time Felisa A. Smith James H. Brown John P. Haskell S. Kathleen Lyons John Alroy See next page for additional authors Follow this and additional works at: http://digitalrepository.unm.edu/biol_fsp Part of the Biology Commons Recommended Citation Smith, Felisa A.; James H. Brown; John P. Haskell; S. Kathleen Lyons; John Alroy; Eric L. Charnov; Tamar Dayan; Brian J. Enquist; S.K. Morgan Ernest; Elizabeth A. Hadly; David Jablonski; Kate E. Jones; Dawn M. Kaufman; Pablo A. Marquet; Brian A. Maurer; Karl J. Niklas; Warren P. Porter; Kaustuv Roy; Bruce Tiffney; and Michael R. Willig. "Similarity of Mammalian Body Size across the Taxonomic Hierarchy and across Space and Time." 163, 5 (2004). http://digitalrepository.unm.edu/biol_fsp/82 This Article is brought to you for free and open access by the Scholarly Communication - Departments at UNM Digital Repository. It has been accepted for inclusion in Biology Faculty & Staff ubP lications by an authorized administrator of UNM Digital Repository. For more information, please contact [email protected]. Authors Felisa A. Smith, James H. Brown, John P. Haskell, S. Kathleen Lyons, John Alroy, Eric L. Charnov, Tamar Dayan, Brian J. Enquist, S.K. Morgan Ernest, Elizabeth A. Hadly, David Jablonski, Kate E. Jones, Dawn M. Kaufman, Pablo A. Marquet, Brian A. Maurer, Karl J. Niklas, Warren P. Porter, Kaustuv Roy, Bruce Tiffney, and Michael R. Willig This article is available at UNM Digital Repository: http://digitalrepository.unm.edu/biol_fsp/82 vol. -

Aka "Miller Moth"
Questions and Answers about Miller Moths by Whitney Cranshaw and Frank Peairs Colorado State University Extension Entomologists Prediction of Nuisance Problems with Army Cutworm (a.k.a. "Miller Moth") for April 25, 2014 There have been several reports that the larvae of the army cutworm are unusually abundant this spring on the plains of northeastern Colorado and adjacent areas of Kansas and Nebraska. This poses risk to infested crops, and some treatments are being made in wheat and alfalfa. However, army cutworm numbers are also a good predictor of the population size of the adults (“miller moths”) to be expected as these insects complete development and make their annual migration to the higher elevations. Based on the stage of cutworm larvae present, adults likely will begin to appear in about a month and a half, but the peak of the migration will not pass through the northern Front Range until a bit later (end of May, early June). The numbers of caterpillars present suggest that the numbers of adults migrating across northeastern Colorado will be well above average. Drought conditions that persist in southeastern Colorado apparently have decreased populations of army cutworms in that part of the state. There are no indications that army cutworms, and the adult “miller moths”, originating out of southeastern Colorado will be unusually abundant this year; an average, perhaps somewhat below average, number of “miller moths” may be seen in that part of the state. However, those that are present will likely concentrate in irrigated areas where there are blooming plants in late May and June. -

REPORT on APPLES – Fruit Pathway and Alert List
EU project number 613678 Strategies to develop effective, innovative and practical approaches to protect major European fruit crops from pests and pathogens Work package 1. Pathways of introduction of fruit pests and pathogens Deliverable 1.3. PART 5 - REPORT on APPLES – Fruit pathway and Alert List Partners involved: EPPO (Grousset F, Petter F, Suffert M) and JKI (Steffen K, Wilstermann A, Schrader G). This document should be cited as ‘Wistermann A, Steffen K, Grousset F, Petter F, Schrader G, Suffert M (2016) DROPSA Deliverable 1.3 Report for Apples – Fruit pathway and Alert List’. An Excel file containing supporting information is available at https://upload.eppo.int/download/107o25ccc1b2c DROPSA is funded by the European Union’s Seventh Framework Programme for research, technological development and demonstration (grant agreement no. 613678). www.dropsaproject.eu [email protected] DROPSA DELIVERABLE REPORT on Apples – Fruit pathway and Alert List 1. Introduction ................................................................................................................................................... 3 1.1 Background on apple .................................................................................................................................... 3 1.2 Data on production and trade of apple fruit ................................................................................................... 3 1.3 Pathway ‘apple fruit’ ..................................................................................................................................... -

Moths of Alaska
Zootaxa 3571: 1–25 (2012) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2012 · Magnolia Press Article ISSN 1175-5334 (online edition) urn:lsid:zoobank.org:pub:C1B7C5DB-D024-4A3F-AA8B-582C87B1DE3F A Checklist of the Moths of Alaska CLIFFORD D. FERRIS1, JAMES J. KRUSE2, J. DONALD LAFONTAINE3, KENELM W. PHILIP4, B. CHRISTIAN SCHMIDT5 & DEREK S. SIKES6 1 5405 Bill Nye Avenue, R.R.#3, Laramie, WY 82070. Research Associate: McGuire Center for Lepidoptera and Biodiversity, Florida Museum of Natural History, University of Florida, Gainesville, FL; C. P. Gillette Museum of Arthropod Diversity, Colorado State Uni- versity, Ft. Collins, CO. cdferris @uwyo.edu 2 USDA Forest Service, State & Private Forestry, Forest Health Protection, Fairbanks Unit, 3700 Airport Way, Fairbanks, AK; Research Associate: University of Alaska Museum, Fairbanks, AK. [email protected] 3 Canadian National Collection of Insects, Arachnids, and Nematodes, Biodiversity Program, Agriculture and Agri-Food Canada, K. E. Neatby Bldg., 960 Carling Ave., Ottawa, Ontario, Canada K1A 0C6. [email protected] 4 1590 Becker Ridge Rd., Fairbanks, AK, 99709. Senior Research Scientist, Institute of Arctic Biology, University of Alaska, Fairbanks, AK; Research Associate: University of Alaska Museum, Fairbanks, AK; National Museum of Natural History, Smithsonian Institution, Washington, DC. [email protected] 5 Canadian Food Inspection Agency, Canadian National Collection of Insects, Arachnids, and Nematodes, Biodiversity Program, Agriculture and Agri-Food Canada, K. E. Neatby Bldg., 960 Carling Ave., Ottawa, Ontario, Canada K1A 0C6. [email protected] 6 University of Alaska Museum, 907 Yukon Drive, Fairbanks, AK 99775-6960. [email protected] Abstract This article represents the first published complete checklist of the moth taxa, resident and occasional, recorded to date for Alaska. -

Insects and Other Terrestrial Arthropods from the Leeward Hawaiian Islands1 Most Recent Immigrant Insects Now Known from The
CORE Metadata, citation and similar papers at core.ac.uk Provided by ScholarSpace at University of Hawai'i at Manoa Vol. XIX, No. 2, September, 1966 157 Insects and Other Terrestrial Arthropods from the Leeward Hawaiian Islands1 John W. Beardsley UNIVERSITY OF HAWAII, HONOLULU, HAWAII INTRODUCTION The Leeward Hawaiian Islands comprise a chain of small rocky islets, and coral atolls which extend west-northwest of Kauai. Nihoa, the nearest, is about 150 miles from Kauai, while Kure, the furthermost, is some 1,150 miles away (see map, p. 158). All Leeward Islands except Midway and Kure are now a part of the Hawaiian Islands National Wildlife Refuge administered by the U.S. Fish and Wildlife Service. This paper summarizes results of recent entomological field work in these islands, and attempts to update the existing lists of insects and other terrestrial arthropods known. The terrestrial arthropod fauna of these islands is a mixture of endemic or indigenous elements and recently, adventive forms. The numbers of endemic species are greatest on the two relatively undisturbed southeastern volcanic islands of Nihoa and Necker, and apparently have disappeared largely from the more northwesterly atolls where, in most cases, the original vegetation has changed drastically in the past 100 years or so. Extinction of native plants and endemic insects has been documented fairly well for Laysan fChristophersen & Caum, 1931, Butler & Usinger, 1963a). Un fortunately, less is known about the original biota of the other atolls. Most recent immigrant insects now known from the Leeward Islands occur also on the larger inhabited islands of Hawaii; however, two species could become serious crop pests should they spread into agricultural areas of the state.