Kuang Et Al. 2008
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abstract Book Progeo 2Ed 20
Abstract Book BUILDING CONNECTIONS FOR GLOBAL GEOCONSERVATION Editors: G. Lozano, J. Luengo, A. Cabrera Internationaland J. Vegas 10th International ProGEO online Symposium ABSTRACT BOOK BUILDING CONNECTIONS FOR GLOBAL GEOCONSERVATION Editors Gonzalo Lozano, Javier Luengo, Ana Cabrera and Juana Vegas Instituto Geológico y Minero de España 2021 Building connections for global geoconservation. X International ProGEO Symposium Ministerio de Ciencia e Innovación Instituto Geológico y Minero de España 2021 Lengua/s: Inglés NIPO: 836-21-003-8 ISBN: 978-84-9138-112-9 Gratuita / Unitaria / En línea / pdf © INSTITUTO GEOLÓGICO Y MINERO DE ESPAÑA Ríos Rosas, 23. 28003 MADRID (SPAIN) ISBN: 978-84-9138-112-9 10th International ProGEO Online Symposium. June, 2021. Abstracts Book. Editors: Gonzalo Lozano, Javier Luengo, Ana Cabrera and Juana Vegas Symposium Logo design: María José Torres Cover Photo: Granitic Tor. Geosite: Ortigosa del Monte’s nubbin (Segovia, Spain). Author: Gonzalo Lozano. Cover Design: Javier Luengo and Gonzalo Lozano Layout and typesetting: Ana Cabrera 10th International ProGEO Online Symposium 2021 Organizing Committee, Instituto Geológico y Minero de España: Juana Vegas Andrés Díez-Herrero Enrique Díaz-Martínez Gonzalo Lozano Ana Cabrera Javier Luengo Luis Carcavilla Ángel Salazar Rincón Scientific Committee: Daniel Ballesteros Inés Galindo Silvia Menéndez Eduardo Barrón Ewa Glowniak Fernando Miranda José Brilha Marcela Gómez Manu Monge Ganuzas Margaret Brocx Maria Helena Henriques Kevin Page Viola Bruschi Asier Hilario Paulo Pereira Carles Canet Gergely Horváth Isabel Rábano Thais Canesin Tapio Kananoja Joao Rocha Tom Casadevall Jerónimo López-Martínez Ana Rodrigo Graciela Delvene Ljerka Marjanac Jonas Satkünas Lars Erikstad Álvaro Márquez Martina Stupar Esperanza Fernández Esther Martín-González Marina Vdovets PRESENTATION The first international meeting on geoconservation was held in The Netherlands in 1988, with the presence of seven European countries. -

A Journal on Taxonomic Botany, Plant Sociology and Ecology
A JOURNAL ON TAXONOMIC BOTANY, LIPI PLANT SOCIOLOGY AND ECOLOGY 12(4) REINWARDTIA A JOURNAL ON TAXONOMIC BOTANY, PLANT SOCIOLOGY AND ECOLOGY Vol. 12(4): 261 - 337, 31 March 2008 Editors ELIZABETH A. WIDJAJA, MIEN A. RIFAI, SOEDARSONO RISWAN, JOHANIS P. MOGEA Correspondece on The Reinwardtia journal and subscriptions should be addressed to HERBARIUM BOGORIENSE, BIDANG BOTANI, PUSAT PENELITIAN BIOLOGI - LIPI, BOGOR, INDONESIA REINWARDTIA Vol 12, Part 4, pp: 285 - 288 NOTES ON MALESIAN NAUCLEEAE Received September 26, 2007; accepted November 5, 2007. C.E.RIDSDALE Nationaal Herbarium Nederland, Universiteit Leiden Branch, P.O. Box 9514, 2300 RA Leiden, The Netherlands. E-mail: [email protected] ABSTRACT RIDSDALE, C.E. 2008. Notes on Malesian Neonaucleea. Reinwardtia 12(4): 285 – 288 — Neonauclea pseudoborneensis, Neonauclea subsessilis and Myrmeconauclea surianii are described as new species. Sarcocephalis fluviatilis Elmer is reinstated as a variety of Myrmeconauclea strigosa. The loss of a large number of type specimens formerly in L is reported. Keyword: Malesia, Neonauclea pseudoborneensis, Neonauclea subsessilis, Myrmeconauclea surianii, Sarcocephalis fluviatilis, Myrmeconauclea strigosa ABSTRAK RIDSDALE, C.E. 2008. Catatan pada Neonaucleea Malesia. Reinwardtia 12(4): 282 – 288 — Neonauclea pseudoborneensis, Neonauclea subsessilis dan Myrmeconauclea surianii diuraikan sebagai jenis baru. Sarcocephalis fluviatilis Elmer direklasifikasi sebagai varietas Myrmeconauclea strigosa. Hilangnya sejumlah tipe specimen yang semula ada di L juga dilaporkan. Kata kunci: Malesia, Neonauclea pseudoborneensis, Neonauclea subsessilis, Myrmeconauclea surianii, Sarco- cephalis fluviatilis, Myrmeconauclea strigosa NEONAUCLEA conicis ochraceis papillatis. Corolla infundibularis 11- 14 mm longa, glabra, lobis ovatis. Stylus per 8-12 mm Since the published revisions of Naucleeae exsertus. Capitula fructifera 30-35 mm diam., fructibus 8 (Ridsdale 1978) and Neonauclea (Ridsdale 1989) mm longis. -

Field Release of the Leaf-Feeding Moth, Hypena Opulenta (Christoph)
United States Department of Field release of the leaf-feeding Agriculture moth, Hypena opulenta Marketing and Regulatory (Christoph) (Lepidoptera: Programs Noctuidae), for classical Animal and Plant Health Inspection biological control of swallow- Service worts, Vincetoxicum nigrum (L.) Moench and V. rossicum (Kleopow) Barbarich (Gentianales: Apocynaceae), in the contiguous United States. Final Environmental Assessment, August 2017 Field release of the leaf-feeding moth, Hypena opulenta (Christoph) (Lepidoptera: Noctuidae), for classical biological control of swallow-worts, Vincetoxicum nigrum (L.) Moench and V. rossicum (Kleopow) Barbarich (Gentianales: Apocynaceae), in the contiguous United States. Final Environmental Assessment, August 2017 Agency Contact: Colin D. Stewart, Assistant Director Pests, Pathogens, and Biocontrol Permits Plant Protection and Quarantine Animal and Plant Health Inspection Service U.S. Department of Agriculture 4700 River Rd., Unit 133 Riverdale, MD 20737 Non-Discrimination Policy The U.S. Department of Agriculture (USDA) prohibits discrimination against its customers, employees, and applicants for employment on the bases of race, color, national origin, age, disability, sex, gender identity, religion, reprisal, and where applicable, political beliefs, marital status, familial or parental status, sexual orientation, or all or part of an individual's income is derived from any public assistance program, or protected genetic information in employment or in any program or activity conducted or funded by the Department. (Not all prohibited bases will apply to all programs and/or employment activities.) To File an Employment Complaint If you wish to file an employment complaint, you must contact your agency's EEO Counselor (PDF) within 45 days of the date of the alleged discriminatory act, event, or in the case of a personnel action. -

A Comprehensive Review Article on Haridru (Adina Cordifolia Hook F)
wjpmr, 2021,7(9), 128 – 132. SJIF Impact Factor: 5.922 WORLD JOURNAL OF PHARMACEUTICAL Review Article Ritu et al. World Journal of Pharmaceutical and Medical Research AND MEDICAL RESEARCH ISSN 2455-3301 www.wjpmr.com WJPMR A COMPREHENSIVE REVIEW ARTICLE ON HARIDRU (ADINA CORDIFOLIA HOOK F) Dr. Ritu*1, Naveen Kumar2, Dr. Kulbhushan3 and Dr. Satya Dev Pandey4 1Assistant Professor in Department of Dravyaguna Vigyana, at DBACH, Mandi Gobindgarh, Punjab. 2B.A.M.S. Student of 2nd year at DBACH, Mandi Gobindgarh, Punjab. 3Professor in Department of Swathvritta, at DBACH, Mandi Gobindgarh, Punjab. 4Professor in Department of Kaya Chikitsa, at DBACH, Mandi Gobindgarh, Punjab. *Corresponding Author: Dr. Ritu Assistant Professor in Department of Dravyaguna Vigyana, at DBACH, Mandi Gobindgarh, Punjab. Article Received on 16/06/2021 Article Revised on 06/07/2021 Article Accepted on 26/07/2021 ABSTRACT Ayurveda a deep ocean with lots of medicinal gems, whether these are known or unknown. Some of its gems are controversial due to their morphological structure and lack of knowledge. Here we are trying to describe Haridru as a separate drug with its medicinal importance. As we know Ayurveda is a life science which deals with about every Dravya present in universe. Haridru is a plant having medicinal importance in Ayurveda but generally explains as a variety of Kadamba or Kadamba itself in many classical texts. We are trying to compile the data which proves that Haridru is a separate drug neither Kadamba nor its variety on the basis of its morphology, properties & actions as per Ayurveda. This study may be helpful for further researchers for conducting their studies because it‟s controversial kind of drug having not much research on this. -

Medicinal Practices of Sacred Natural Sites: a Socio-Religious Approach for Successful Implementation of Primary
Medicinal practices of sacred natural sites: a socio-religious approach for successful implementation of primary healthcare services Rajasri Ray and Avik Ray Review Correspondence Abstract Rajasri Ray*, Avik Ray Centre for studies in Ethnobiology, Biodiversity and Background: Sacred groves are model systems that Sustainability (CEiBa), Malda - 732103, West have the potential to contribute to rural healthcare Bengal, India owing to their medicinal floral diversity and strong social acceptance. *Corresponding Author: Rajasri Ray; [email protected] Methods: We examined this idea employing ethnomedicinal plants and their application Ethnobotany Research & Applications documented from sacred groves across India. A total 20:34 (2020) of 65 published documents were shortlisted for the Key words: AYUSH; Ethnomedicine; Medicinal plant; preparation of database and statistical analysis. Sacred grove; Spatial fidelity; Tropical diseases Standard ethnobotanical indices and mapping were used to capture the current trend. Background Results: A total of 1247 species from 152 families Human-nature interaction has been long entwined in has been documented for use against eighteen the history of humanity. Apart from deriving natural categories of diseases common in tropical and sub- resources, humans have a deep rooted tradition of tropical landscapes. Though the reported species venerating nature which is extensively observed are clustered around a few widely distributed across continents (Verschuuren 2010). The tradition families, 71% of them are uniquely represented from has attracted attention of researchers and policy- any single biogeographic region. The use of multiple makers for its impact on local ecological and socio- species in treating an ailment, high use value of the economic dynamics. Ethnomedicine that emanated popular plants, and cross-community similarity in from this tradition, deals health issues with nature- disease treatment reflects rich community wisdom to derived resources. -

CO-CHAIRS' SUMMARY REPORT 2ND ASEAN REGIONAL FORUM WORKSHOP of NATIONAL MARITIME SINGLE POINTS of CONTACT Kuala Lumpur, Malay
FINAL CO-CHAIRS’ SUMMARY REPORT 2ND ASEAN REGIONAL FORUM WORKSHOP OF NATIONAL MARITIME SINGLE POINTS OF CONTACT Kuala Lumpur, Malaysia, 27-29 August 2018 INTRODUCTION 1. The 2nd ASEAN Regional Forum (ARF) Workshop of National Maritime Single Points of Contact (NMSPOC) was held from 27 to 29 August 2018 in Kuala Lumpur, Malaysia, as approved by the 24th ARF Ministerial Meeting on 7 August 2017 in Manila, the Philippines. It served as a follow-up to the previous ARF NMSPOC Workshops, including the ARF NMSPOC Workshop held from 28 to 29 April 2016 in Cebu City, the Philippines, during which it was agreed that the NMSPOC concept has merit in the ASEAN context and should be pursued further. Therefore, the purpose of this 2nd Workshop was to further define the idea of a NMSPOC by identifying and sharing best practices in inter-agency cooperation, coordination, and information sharing. 2. The Workshop was co-chaired by Dr. Adina Kamarudin, Director-General, Maritime Affairs Department, Ministry of Foreign Affairs, Malaysia; Dr. Christopher Merritt, Maritime Technical Advisor, the United States Mission to ASEAN in Jakarta; and Commander Christopher Waters, Regional Director of South East Asia, Australian Border Force in Jakarta. 3. Representatives from ARF Member States (Cambodia, Indonesia, Malaysia, Myanmar, the Philippines, Thailand and Vietnam) as well as Australia, Canada, China, Japan, Pakistan, Russia, Timor Leste, the United States and the ASEAN Secretariat participated in the Workshop. The Programme of Activities appears as Annex 1, and the List of Participants as Annex 2. SESSION 1 – OPENING CEREMONY : CO-CHAIRS’ INTRODUCTION AND WELCOMING REMARKS Page 1 of 11 FINAL 4. -

International Journal of Pharmacy & Life Sciences
Explorer Research Article [Maitreya 6(5): May, 2015:4476-4480] CODEN (USA): IJPLCP ISSN: 0976-7126 INTERNATIONAL JOURNAL OF PHARMACY & LIFE SCIENCES (Int. J. of Pharm. Life Sci.) An overview of Ethnomedicinal plants of Family Rubiaceae from Sabarmati River of Gujarat state, India Bharat B. Maitreya Sir P.P.Institute of Science, Maharaja Krishnakumarsinhji Bhavnagar University, Bhavnagar, (Gujarat) - India Abstract An overview of ethnomedicinal values of plant species in family Rubiaceae , which are grow in the area of Sabarmati river . Plant exploration was conducted to determine plant species of family Rubiaceae. Taxonomic position of these plant species is described in various available Floras. Plant species of family Rubiaceae from Sabarmati riverbed–riverside area have been listed systematically which counts 13 species of 12 genera ,09 wild plants species and 05 cultivated plant species. Most of the plant species and their plant parts have ethnomedicinal values and its utilize in different kind of diseases and uses as different form. Key-Words: Ethnomedicinal, Rubiaceae, Sabarmati river Introduction Rubiaceae family is dicot angiosperm and having 340 Study area Genera and 4000 species found in tropical and sub The geographical situation of the Sabarmati tropical. It includes herbs, shrub, tree and rarely river is between 22° 30’ to 24° 30’ North twiners. Life span is pereenials and annuals. This latitude and 72° 30’ to 73° 30’ East longitude. It family easily recognized by their interpetiolar stipules originates from Arvalli hills, near Vekaria in [4] Ethnomedicinal plants - drug yielding plants have Rajasthan State and enters in the Gujarat state at been used since ancient times for the treatment of the boundary of the Sabarkantha district .It human ailments. -

Anthocephalus Cadamba (Roxb.) Miq
Pharmacogn. Res. REVIEW ARTICLE A multifaceted peer reviewed journal in the field of Pharmacognosy and Natural Products www.phcogres.com | www.phcog.net “Haripriya” God’s Favorite: Anthocephalus cadamba (Roxb.) Miq. ‑ At a Glance Sumanta Mondal, Kausik Bhar1, Ashes Sinha Mahapatra, Joy Mukherjee, Prasenjit Mondal2, Syed Tazib Rahaman, Aishwarya P. Nair Department of Pharmaceutical Chemistry, Institute of Pharmacy, GITAM (Deemed to be University), Visakhapatnam, Andhra Pradesh, 1Department of Pharmaceutical Chemistry, Bengal School of Technology, Hooghly, West Bengal, 2Department of Pharmaceutical Chemistry and Analysis, Vaageswari College of Pharmacy, Karimnagar, Telangana, India ABSTRACT The Kadam tree is highly regarded as religiously and culturally in India being sacred to Lord Krishna, and hence, the tree is also known as Haripriya, God’s favorite. This article provides a detailed review of Anthocephalus cadamba (Roxb) Miq. (family – Rubiaceae) that covers taxonomical classification, vernacular names, geographical distribution, botanical description, ethnobotanical information, pharmacological studies, and phytochemistry. Several parts of this plant have a number of traditional applications for treating humanity, which includes mouth ulcer, subdermal inflammatory deposits, stomatitis, fever, gastric disturbance, astringent, febrifuge, antiseptic, diuretics, anemia, uterine complaints, increase breast milk in lactating women, improvement of semen quality in men, nanotechnology, and agroforestry. The plant parts produce various pharmacological -

Pollen Morphology of the Tribes Naucleeae and Hymenodictyeae (Rubiaceae – Cinchonoideae) and Its Phylogenetic Significance
Blackwell Publishing LtdOxford, UKBOJBotanical Journal of the Linnean Society0024-40742007 The Linnean Society of London? 2007 1533 329341 Original Article POLLEN MORPHOLOGY OF NAUCLEEAE AND HYMENODICTYEAEJ. VERELLEN ET AL . Botanical Journal of the Linnean Society, 2007, 153, 329–341. With 24 figures Pollen morphology of the tribes Naucleeae and Hymenodictyeae (Rubiaceae – Cinchonoideae) and its phylogenetic significance JEF VERELLEN1, STEVEN DESSEIN1,3, SYLVAIN G. RAZAFIMANDIMBISON2, Downloaded from https://academic.oup.com/botlinnean/article/153/3/329/2420406 by guest on 24 September 2021 ERIK SMETS FLS1,4 and SUZY HUYSMANS1* 1Laboratory of Plant Systematics, Institute of Botany and Microbiology, K.U.Leuven, Kasteelpark Arenberg 31, BE-3001 Leuven, Belgium 2National Botanic Garden of Belgium, Domein van Bouchout, BE-1860 Meise, Belgium 3The Bergius Foundation at the Royal Swedish Academy of Sciences, P.O. Box 50017, SE-104 05 Stockholm, Sweden 4National Herbarium of the Netherlands, Leiden University Branch, P.O. Box 9514, 2300 RA Leiden, the Netherlands Received March 2006; accepted for publication September 2006 The tribe Naucleeae has recently been recircumscribed on the basis of both morphological and molecular [rbcL, trnT- F, internal transcribed spacer (ITS)] evidence, and has been found to be the sister group of the tribe Hymenodictyeae Razafim. & B. Bremer. In order to find pollen morphological support for this new classification, the pollen and orb- icules of 65 species, representing 23 Naucleeae and the two Hymenodictyeae genera, were investigated by scanning electron and light microscopy. Naucleeae pollen is very small (< 20 µm) to small (20–30 µm) and its shape in equatorial view is suboblate to spheroidal or, more rarely, subprolate. -
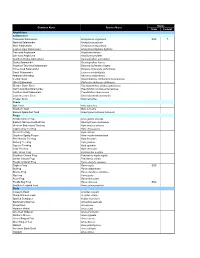
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

Buttonbush, Cephalanthus Occidentalis
Buttonbush, Cephalanthus occidentalis Buttonbush is a deciduous, multi-stemmed, loose shrub or small tree in the coffee family (Rubiaceae). Generally reaching no more than 12 feet in height, the plant is frequently wider than it is tall. It is native to North America from Nova Scotia and Ontario south to Mexico. It is found in the Florida Everglades. A key characteristic of the Buttonbush is its flower heads which consist of fragrant, tiny, tubular, white flowers compacted into 1-inch diameter spheres. Each flower has a projecting style which gives the flower head a pincushion-like appearance. The long- lasting blooms (June – August) give way to hard, spherical clusters of nutlets that resemble old-time dress buttons; hence its common name. The fruit matures in fall and can stay on the plant through winter, providing interest for the garden and food for wildlife. The beautiful dark green, glossy foliage is another ornamental feature of Buttonbush. The leaves, which emerge late in spring, are opposite or in whorls of three, ovate in shape, 2-6 inches long, 1-3 inches wide, with a smooth edge and short petiole. The leaves turn yellow- green in the fall. Other identifying features of Buttonbush are prominent lenticels on coarse stems, absent terminal buds, and pith that is solid and light brown. Buttonbush occurs as a non-dominant midstory species in mixed riparian forests, pond or stream margins, and swamps. They prefer moist, humusy soils and full to partial sunlight. Buttonbush in the wild is an indicator of an area’s wetland status. The plant will tolerate water depths up to three feet and long durations of flooding. -

Comparison of Mimosine Content and Nutritive Values of Neolamarckia Cadamba and Leucaena Leucocephala with Medicago Sativa As Forage
INTERNATIONAL JOURNAL OF SCIENTIFIC & TECHNOLOGY RESEARCH VOLUME 3, ISSUE 8, AUGUST 2014 ISSN 2277-8616 Comparison Of Mimosine Content And Nutritive Values Of Neolamarckia Cadamba And Leucaena Leucocephala With Medicago Sativa As Forage Quality Index Mohamed Zaky Zayed, Mohamed Abdallah Zaki, Fasihuddin Badruddin Ahmad, Wei-Seng Ho, Shek-Ling Pang Abstract: A study was conducted to determine the mimosine content and the nutritive values of Neolamarckia cadamba and Leucaena leucocephala in comparison to Medicago saliva (alfalfa hay) as forage quality index. A total of 22 N. cadamba and 35 L. leucocephala seedlings were analyzed to determine the mimosine content after 6 months of planting. It was noted that the mimosine content was highest in L. leucocephala (1.6%) and lowest in N. cadamba (0.03%) in comparison to M. sativa which has no mimosine content. Crude protein content was 23.48%, 20.90% and 14.83% for L. leucocephala, N. cadamba and M. sativa, respectively. The crude fiber was maximum in M. sativa (27.23%) and minimum in L. leucocephala (18.77%). Crude protein, crude fat, gross energy, protein to energy (P/E) ratio, organic matter and total ash in N. cadamba was higher compared to M. sativa. L. leucocephala was lower in nitrogen free extract, crude fiber and total ash compared to N. cadamba. Results from this study clearly indicate that N. cadamba has high forage quality and comparable to the traditional L. leucocephala and M. sativa as forage for ruminant and non-ruminants. Index Terms: Neolamarckia cadamba, Leucaena leucocephala, Medicago sativa, mimosine, nutritive value, forage quality index ———————————————————— 1 INTRODUCTION The need to develop cheap and readily available alternative Leucaena leucocephala or locally known as petai belalang feeding materials to support livestock growth has become belongs to family Leguminosae.