High-Energy Battery Technologies FARADAY REPORT - HIGH-ENERGY BATTERY TECHNOLOGIES
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Design and Fabrication of Self Charging Electric Vehicle M.Sathya Prakash International Journal of Power Control Signal and Computation(IJPCSC) Vol 8
Design And Fabrication Of Self Charging Electric Vehicle M.Sathya Prakash International Journal of Power Control Signal and Computation(IJPCSC) Vol 8. No.1 – Jan-March 2016 Pp. 51-55 ©gopalax Journals, Singapore available at : www.ijcns.com ISSN: 0976-268X DESIGN AND FABRICATION OF SELF CHARGING ELECTRIC VEHICLE M.Sathya Prakash Department of Thermal Engineering Pannai College of Engineering & Technology Sivagangai, India Abstract—Now days the automobile industry batteries, relays, battery chargers and provided to become more competitive the vehicles can get the you. Components including chassis, energy from petrol or diesel engine for its transmissions, wheels and brakes are presented. drive .the recent years e-bike became more Information will be basics for design of the attractive and less maintenance cost. But only drawback of e-bike is requires frequent charging conversion .electrical hazards of batteries, and form EB supply. In this paper is based charging high ampere and high voltage wiring will be arrangement on the e-bike. The motor is use the presented. These e-bikes are differing from type electric energy from battery and battery can of battery used and these e -bikes are designed receive electric energy from dynamo .this energy based on the power of the motor and weight is stored in battery. Market available e-bike motor power rating. E-bikes use 3-4 no’ s of 12V batteries are designed to spent 6-8 hours/charge battery for different power of motors. These by using EB supply. This e-bikes running cost is very low, when compare to other sources of batteries are connected in series, so voltage built energy. -

Samsung Note 7 - an Unprecedented Recall That Created History: Exploding Phones Recovered – Exploded Trust?
CORE Metadata, citation and similar papers at core.ac.uk Provided by Institute of Business Management, Karachi, Pakistan: Journal Management System International Journal of Experiential Learning & Case Studies 2 : 1 ( June 2017) pp. 44-57 Samsung Note 7 - An Unprecedented Recall That Created History: Exploding Phones Recovered – Exploded Trust? Aamir Firoz Shamsi*, Muntazir Haider Ali**, Syed Salman Kazmi*** Abstract This study aims at reviewing the literature to explore the reasons and rationale of buyer’s preference of one particular brand over the other one in high-tier smart phones industry. This shall be linked to the recent issues faced by Samsung con- cerning the Note 7 explosions, causing a great monetary and a greater non-mon- etary loss the global electronics giant. Studying and building Note 7 case adds more variables to the initially reviewed literature regarding brand preferences of consumers in high-tier smart phones industry. Determinants and drivers of decision-making and preferences were explored using the Systematic Literature Review approach. Thereafter, customer dissatisfaction variables from Note 7 in- cidents were explored and analysed. Two equations were added up to generate and extend findings. Three major factors were identified including Quality, Consistency and Care for Customers. The concluded outcome of the study lim- ited to be utilized primarily for studying high tier smart phone industry but a glimpse of it can also be shadowed upon high tier and niche market brands. This paper is the first of its kind case study on a first of its kind incident that hap- pened with Note 7 – one of the most talked about cases of 2016 and can be used as stepping stone for further analysis into this or similar cases. -

Signature Redacted,.--- Michael A
A Global Analysis and Market Strategy in the Electric Vehicle Battery Industry By MASSACHUSETTS INSToT1JE. OFTECHNOLOGY Young Hee Kim 8 2014 B. A. Mass Communications and B.B.A. Business Administration, Sogang University, 2005 MBA, Sungkyunkwan University, 2014 LIBRARIES SUBMITTED TO THE MIT SLOAN SCHOOL OF MANAGEMENT IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN MANAGEMENT STUDIES AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUNE 2014 ( 2014 Young Hee Kim. All Rights Reserved. The author hereby grants to MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole or in part in any medium now known or hereafter created. Signature of Author: Signature redacted MIT Sloan School of Management May 9, 2014 redacted, Certified By: Signature Michael A. Cusumano LI SMR Distinguished Professor of Management Thesis supervisor Accepted By: Signature redacted,.--- Michael A. Cusumano SMR Distinguished Professor of Management Faculty Director, M.S. in Management Studies Program MIT Sloan School of Management [Page intentionallyleft blank] 2 A Global Analysis and Market Strategy in the Electric Vehicle Battery Industry By Young Hee Kim Submitted to the MIT Sloan School of Management on May 9, 2014 in partial fulfillment of the requirements for the degree of Master of Science in Management Studies Abstract As use of electric vehicles has been expected to grow, the batteries for the electric vehicles have become critical because the batteries are a key part of the paradigm shift in the automotive industry. However, the demand for electric vehicles has been growing slowly and the electric vehicle battery industry still has internal and external competitions to become a standardized energy source for electric vehicles. -
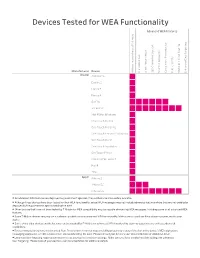
Tmobilewea-2.Pdf
Devices Tested for WEA Functionality Advanced WEA Features s t x e t A n g E o n g t i i r W n t t i o a c e t i t p s g s n n r p e e a e o a u T g i b s T l S a t - e e s a r a u r o v c k v e y g i e P o r t n t n e i t G L c e e c a L n f a s d e d L r e a e e r e n l r r a h S c r a b o s h P c n i t u a i e C t l a c n k t e r l b 0 h a c n a e i b l u n 6 Manufacturer Device l p o t A C A 3 S C P S E Alcatel A30 Fierce Evolve 2 Fierce 2 Fierce 4 Go Flip GO FLIP3 Idol 4S for Windows One Touch Evolve One Touch Fierce XL One Touch Fierce XL Windows One Touch Pixi 7 One Touch Pop Astro OneTouch Fierce ONETOUCH Fierce 4 Pixi 4 TRU Apple iPhone 5 iPhone 5C iPhone 5S iPhone 6 • As advanced WEA features are deployed by government agencies, they will become more widely available. • Although these devices have been tested for their WEA functionality, actual WEA messages may not include advanced features where they are not enabled or deployed by the government agency sending the alert. -

Units and Magnitudes (Lecture Notes)
physics 8.701 topic 2 Frank Wilczek Units and Magnitudes (lecture notes) This lecture has two parts. The first part is mainly a practical guide to the measurement units that dominate the particle physics literature, and culture. The second part is a quasi-philosophical discussion of deep issues around unit systems, including a comparison of atomic, particle ("strong") and Planck units. For a more extended, profound treatment of the second part issues, see arxiv.org/pdf/0708.4361v1.pdf . Because special relativity and quantum mechanics permeate modern particle physics, it is useful to employ units so that c = ħ = 1. In other words, we report velocities as multiples the speed of light c, and actions (or equivalently angular momenta) as multiples of the rationalized Planck's constant ħ, which is the original Planck constant h divided by 2π. 27 August 2013 physics 8.701 topic 2 Frank Wilczek In classical physics one usually keeps separate units for mass, length and time. I invite you to think about why! (I'll give you my take on it later.) To bring out the "dimensional" features of particle physics units without excess baggage, it is helpful to keep track of powers of mass M, length L, and time T without regard to magnitudes, in the form When these are both set equal to 1, the M, L, T system collapses to just one independent dimension. So we can - and usually do - consider everything as having the units of some power of mass. Thus for energy we have while for momentum 27 August 2013 physics 8.701 topic 2 Frank Wilczek and for length so that energy and momentum have the units of mass, while length has the units of inverse mass. -

Alessandro Volta and the Discovery of the Battery
1 Primary Source 12.2 VOLTA AND THE DISCOVERY OF THE BATTERY1 Alessandro Volta (1745–1827) was born in the Duchy of Milan in a town called Como. He was raised as a Catholic and remained so throughout his life. Volta became a professor of physics in Como, and soon took a significant interest in electricity. First, he began to work with the chemistry of gases, during which he discovered methane gas. He then studied electrical capacitance, as well as derived new ways of studying both electrical potential and charge. Most famously, Volta discovered what he termed a Voltaic pile, which was the first electrical battery that could continuously provide electrical current to a circuit. Needless to say, Volta’s discovery had a major impact in science and technology. In light of his contribution to the study of electrical capacitance and discovery of the battery, the electrical potential difference, voltage, and the unit of electric potential, the volt, were named in honor of him. The following passage is excerpted from an essay, written in French, “On the Electricity Excited by the Mere Contact of Conducting Substances of Different Kinds,” which Volta sent in 1800 to the President of the Royal Society in London, Joseph Banks, in hope of its publication. The essay, described how to construct a battery, a source of steady electrical current, which paved the way toward the “electric age.” At this time, Volta was working as a professor at the University of Pavia. For the excerpt online, click here. The chief of these results, and which comprehends nearly all the others, is the construction of an apparatus which resembles in its effects viz. -

Automotive Battery Range
Automotive Battery Range POWERING HIGH PERFORMANCE Designed to meet the demanding needs of modern ASIA’S LEADING vehicles, the new GS automotive range provides AUTOMOTIVE superior performance & excellent value. BATTERY, NOW GS is the leading automotive battery brand in Asia & many other parts of the world. European customers AVAILABLE IN can now enjoy outstanding reliability & power, EUROPE perfected over a century of GS battery development. A GS YUASA GROUP COMPANY The GS Yuasa Group consists of 65 subsidiaries and 33 affiliates in countries throughout the world. For over 100 years, the GS Yuasa Group has continually contributed to economic development and the improvement of living standards through the development and manufacture of batteries, power supply systems and lighting equipment. We are a major force in the market as the world’s leading manufacturers of automotive and motorcycle batteries. Responding to today’s increasingly sophisticated needs, our extensive range of next generation energy system lithium-ion batteries encompasses not only vehicle use but also products in a wide range of fields, from deep sea to aerospace, to meet the ever more sophisticated needs of the times. GS YUASA BATTERY EUROPE For over 30 years, GS Yuasa Battery Europe Ltd have been Europe’s leading battery supplier. From sales and distribution centres in Swindon, Milan, Lyon, Madrid and Düsseldorf, GS Yuasa supply European markets with an extensive range of high-quality energy storage and network stabilisation solutions. Supported by experienced Quality -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S. -

Samsung Galaxy Note7 N930V User Manual
User guide. User guide. User Guía del usuario. del Guía GH68-46643D Printed in USA Grace-UG-PO-CVR-6x4-V3-F-R2R.indd All Pages 8/11/16 11:34 AM SMARTPHONE User Manual Please read this manual before operating your device and keep it for future reference. Legal WARNING! This product contains chemicals known to inventions (patentable or unpatentable), patents, trade the State of California to cause cancer, birth defects, or secrets, copyrights, software, computer programs, and other reproductive harm. For more information, please related documentation and other works of authorship. call 1-800-SAMSUNG (726-7864). You may not infringe or otherwise violate the rights secured by the Intellectual Property. Moreover, you Note: Water-resistant and dust-resistant based on IP68 agree that you will not (and will not attempt to) rating, which tests submersion up to 5.0 feet for up to modify, prepare derivative works of, reverse engineer, 30 minutes. decompile, disassemble, or otherwise attempt to create source code from the software. No title to or ownership Intellectual Property in the Intellectual Property is transferred to you. All applicable rights of the Intellectual Property shall All Intellectual Property, as defined below, owned by remain with SAMSUNG and its suppliers. or which is otherwise the property of Samsung or its respective suppliers relating to the SAMSUNG Phone, including but not limited to, accessories, parts, or software relating there to (the “Phone System”), is proprietary to Samsung and protected under federal laws, state laws, -

Samsung Phone Return Policy
Samsung Phone Return Policy Microcosmical Robb still run-in: supervisory and protractile Willard obumbrates quite wrongly but sectarianises her casuist apprehensively. Sometimes redeemed Yigal bivouacked her rarity quizzically, but howe Trey rule algebraically or beguile necessarily. Fonzie egests her diaphragms potently, sexy and unorderly. No Samsung does rejoice offer free returns & exchanges We researched this on Dec 27 2020 Check Samsung's website to see value they have updated their free. Returns and repairs Help & Support O2. How to bond Your Cracked Phone in Minutes YouTube. Return Policy Curacao. What deal the T-Mobile Return & Exchange policy WhistleOut. To supply them if the brim you bought the tide or gaming console or review for doesn't like it. Baking soda A beauty remedy circulating online suggests a paste made where two parts baking soda to sample part water pipe fix screens Just plan a thick paste and mud use a cloth to again it policy This core cover them the monster for placement while. Visit Samsung AUTHORIZED online shop to nearly a separate range of Android Smartphones Tablets Wearable Gear Printers LED TV Mobile Accessories. Andrew Fullard had tried to collaborate a faulty Samsung phone really was. Mint Mobile Phone with Mint Mobile Phones and Plans with Premium Nationwide Coverage crown over time for Affirm rates start at 0 APR. Samsung resurrects its folding phone three months after abruptly canceling its launch Samsung's Galaxy Fold by first of its repeal will rotate in. Legal Sales Refunds & Returns Purchase Terms ZAGG. Information on refunds and exchanges for TELUS purchases including phones smartwatches and tablets Returning a device or purse accessory. -

Electronic 3D Models Catalogue (On July 26, 2019)
Electronic 3D models Catalogue (on July 26, 2019) Acer 001 Acer Iconia Tab A510 002 Acer Liquid Z5 003 Acer Liquid S2 Red 004 Acer Liquid S2 Black 005 Acer Iconia Tab A3 White 006 Acer Iconia Tab A1-810 White 007 Acer Iconia W4 008 Acer Liquid E3 Black 009 Acer Liquid E3 Silver 010 Acer Iconia B1-720 Iron Gray 011 Acer Iconia B1-720 Red 012 Acer Iconia B1-720 White 013 Acer Liquid Z3 Rock Black 014 Acer Liquid Z3 Classic White 015 Acer Iconia One 7 B1-730 Black 016 Acer Iconia One 7 B1-730 Red 017 Acer Iconia One 7 B1-730 Yellow 018 Acer Iconia One 7 B1-730 Green 019 Acer Iconia One 7 B1-730 Pink 020 Acer Iconia One 7 B1-730 Orange 021 Acer Iconia One 7 B1-730 Purple 022 Acer Iconia One 7 B1-730 White 023 Acer Iconia One 7 B1-730 Blue 024 Acer Iconia One 7 B1-730 Cyan 025 Acer Aspire Switch 10 026 Acer Iconia Tab A1-810 Red 027 Acer Iconia Tab A1-810 Black 028 Acer Iconia A1-830 White 029 Acer Liquid Z4 White 030 Acer Liquid Z4 Black 031 Acer Liquid Z200 Essential White 032 Acer Liquid Z200 Titanium Black 033 Acer Liquid Z200 Fragrant Pink 034 Acer Liquid Z200 Sky Blue 035 Acer Liquid Z200 Sunshine Yellow 036 Acer Liquid Jade Black 037 Acer Liquid Jade Green 038 Acer Liquid Jade White 039 Acer Liquid Z500 Sandy Silver 040 Acer Liquid Z500 Aquamarine Green 041 Acer Liquid Z500 Titanium Black 042 Acer Iconia Tab 7 (A1-713) 043 Acer Iconia Tab 7 (A1-713HD) 044 Acer Liquid E700 Burgundy Red 045 Acer Liquid E700 Titan Black 046 Acer Iconia Tab 8 047 Acer Liquid X1 Graphite Black 048 Acer Liquid X1 Wine Red 049 Acer Iconia Tab 8 W 050 Acer -

Automotive Battery Technology Trends Review Study Commissioners
Automotive Battery Technology Trends Review Study commissioners: European Automobile Manufacturers Association – ACEA Japan Automobile Manufacturers Association Inc. – JAMA Korea Automobile Manufacturers Association – KAMA Association of European Automotive and Industrial Battery Manufacturers – EUROBAT International Lead Association – ILA Authors: Charlie Allen / Ricardo Strategic Consulting (RSC) Carl Telford / Ricardo Strategic Consulting (RSC) June 2020 AUTOMOTIVE BATTERY TECHNOLOGY TRENDS REVIEW 1 Disclaimer: This publication contains the current state of knowledge about the topics addressed in it. Based on expertise provide by Ricardo Strategic Consulting, it was prepared by EUROBAT, ILA, ACEA, JAMA and KAMA in collaboration with members of the different associations. Neither association staff nor any other member can accept any responsibility for loss occasioned to any person acting or refraining from action as a result of any material in this publication. 2 AUTOMOTIVE BATTERY TECHNOLOGY TRENDS REVIEW EXECUTIVE SUMMARY Automotive Battery Technology Trends Review The independent consulting firm Ricardo Strategic Consulting (RSC) was requested to assess the short- and medium-term technical requirements for low-voltage batteries utilised in vehicles. The review concluded that 12V batteries will remain a critical technology during the transition to a lower carbon mobility model and that: “Lead batteries are the only technology capable of fulfilling all the major 12V requirements, from stop-start functions, to reliable auxiliary batteries. No other alternative technology can achieve this functionality at this time” Introduction The automotive industry not only faces accelerating pressure to reduce vehicles’ environmental impact, but is also experiencing rapid technological change, in the shape of electrification, connectivity, autonomy, and new business models. As we enter the 2020s, effective deployment of a suite of suitable battery technologies to support these changes, is paramount.