Geochemistry of Fluvio-Tidal Paleogene Litho-Units of Nsugbe
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

World Bank Document
SFG1692 V36 Hospitalia Consultaire Ltd ENVIRONMENTAL AND SOCIAL MANAGEMENT PLAN (ESMP) Public Disclosure Authorized NNEWICHI GULLY EROSION SITE, NNEWI NORTH LGA, ANAMBRA STATE Public Disclosure Authorized Public Disclosure Authorized Anambra State Nigeria Erosion and Watershed Management Project Public Disclosure Authorized November 2017 Table of Contents List of Plates ..................................................................................................................... v List of Tables .................................................................................................................. vii list of acronyms ............................................................................................................. viii EXECUTIVE SUMMARY ..................................................................................................... ix 1 CHAPTER ONE: INTRODUCTION ................................................................................ 17 1.1 Background ..................................................................................................................... 17 1.2 Hydrology ........................................................................................................................ 18 1.3 Hydrography .................................................................................................................... 19 1.4 Hydrogeology .................................................................................................................. 20 1.5 Baseline Information -

Research Report
1.1 CHAPTER 1 INTRODUCTION Soil erosion is the systematic removal of soil, including plant nutrients, from the land surface by various agents of denudation (Ofomata, 1985). Water being the dominant agent of denudation initiates erosion by rain splash impact, drag and tractive force acting on individual particles of the surface soil. These are consequently transported seizing slope advantage for deposition elsewhere. Soil erosion is generally created by initial incision into the subsurface by concentrated runoff water along lines or zones of weakness such as tension and desiccation fractures. As these deepen, the sides give in or slide with the erosion of the side walls forming gullies. During the Stone Age, soil erosion was counted as a blessing because it unearths valuable treasures which lie hidden below the earth strata like gold, diamond and archaeological remains. Today, soil erosion has become an endemic global problem, In the South eastern Nigeria, mostly in Anambra State, it is an age long one that has attained a catastrophic dimension. This environmental hazard, because of the striking imprints on the landscape, has sparked off serious attention of researchers and government organisations for sometime now. Grove(1951); Carter(1958); Floyd(1965); Ofomata (1964,1965,1967,1973,and 1981); all made significant and refreshing contributions on the processes and measures to combat soil erosion. Gully Erosion is however the prominent feature in the landscape of Anambra State. The topography of the area as well as the nature of the soil contributes to speedy formation and spreading of gullies in the area (Ofomata, 2000);. 1.2 Erosion Types There are various types of erosion which occur these include Soil Erosion Rill Erosion Gully Erosion Sheet Erosion 1.2.1 Soil Erosion: This has been occurring for some 450 million years, since the first land plants formed the first soil. -

CHRISTIANITY of CHRISTIANS: an Exegetical Interpretation of Matt
CHRISTIANITY OF CHRISTIANS: An Exegetical Interpretation of Matt. 5:13-16 And its Challenges to Christians in Nigerian Context. ANTHONY I. EZEOGAMBA Copyright © Anthony I. Ezeogamba Published September 2019 All Rights Reserved: No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording or any information storage or retrieval system, without prior written permission from the copyright owner. ISBN: 978 – 978 – 978 – 115 – 7 Printed and Published by FIDES MEDIA LTD. 27 Archbishop A.K. Obiefuna Retreat/Pastoral Centre Road, Nodu Okpuno, Awka South L.G.A., Anambra State, Nigeria (+234) 817 020 4414, (+234) 803 879 4472, (+234) 909 320 9690 Email: [email protected] Website: www.fidesnigeria.com, www.fidesnigeria.org ii DEDICATION This Book is dedicated to my dearest mother, MADAM JUSTINA NKENYERE EZEOGAMBA in commemoration of what she did in my life and that of my siblings. iii ACKNOWLEDGEMENTS First and foremost, I wish to acknowledge the handiwork of God in my life who is the author of my being. I am grateful to Most Rev. Dr. S.A. Okafor, late Bishop of Awka diocese who gave me the opportunity to study in Catholic Institute of West Africa (CIWA) where I was armed to write this type of book. I appreciate the fatherly role of Bishop Paulinus C. Ezeokafor, the incumbent Bishop of Awka diocese together with his Auxiliary, Most Rev. Dr. Jonas Benson Okoye. My heartfelt gratitude goes also to Bishop Peter Ebele Okpalaeke for his positive influence in my spiritual life. I am greatly indebted to my chief mentor when I was a student priest in CIWA and even now, Most Rev. -
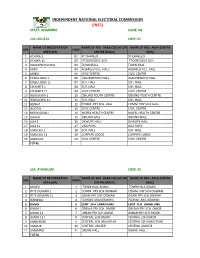
State: Anambra Code: 04
INDEPENDENT NATIONAL ELECTORAL COMMISSION (INEC) STATE: ANAMBRA CODE: 04 LGA :AGUATA CODE: 01 NAME OF REGISTRATION NAME OF REG. AREA COLLATION NAME OF REG. AREA CENTRE S/N CODE AREA (RA) CENTRE (RACC) (RAC) 1 ACHINA 1 01 ST CHARLED ST CHARLED 2 ACHINA 11 02 PTOGRESSIVE SCH. PTOGRESSIVE SCH. 3 AGULEZECHUKWU 03 TOWN HALL TOWN HALL 4 AKPO 04 AGBAELU VILL. HALL AGBAELU VILL. HALL 5 AMESI 05 CIVIC CENTRE CIVIC CENTRE 6 EKWULOBIA 1 06 UMUEZENOFO HALL. UMUEZENOFO HALL. 7 EKWULOBIA 11 07 SCH. HALL SCH. HALL 8 EZENIFITE 1 08 SCH. HALL SCH. HALL 9 EZENIFITE 11 09 CIVIC CENTRE CIVIC CENTRE 10 IGBOUKWU 1 10 OBIUNO YOUTH CENTRE OBIUNO YOUTH CENTRE 11 IGBOUKWU 11 11 SCH. HALL SCH. HALL 12 IKENGA 12 COMM. PRY SCH. HALL COMM. PRY SCH. HALL 13 ISUOFIA 13 CIVIC CENTRE CIVIC CENTRE 14 NKPOLOGWU 14 MOFEL HEALTH CENTRE MOFEL HEALTH CENTRE 15 ORAERI 15 OBIUNO HALL OBIUNO HALL 16 UGA 1 16 OKWUTE HALL OKWUTE HALL 17 UGA 11 17 UGA BOYS UGA BOYS 18 UMUCHU 1 18 SCH. HALL SCH. HALL 19 UMUCHU 11 19 CORPERS LODGE CORPERS LODGE 20 UMOUNA 20 CIVIC CENTRE CIVIC CENTRE TOTAL LGA: AYAMELUM CODE: 02 NAME OF REGISTRATION NAME OF REG. AREA COLLATION NAME OF REG. AREA CENTRE S/N CODE AREA (RA) CENTRE (RACC) (RAC) 1 ANAKU 1 TOWN HALL ANAKU TOWN HALL ANAKU 2 IFITE OGWARI 1 2 COMM. PRY SCH.OGWARI COMM. PRY SCH.OGWARI 3 IFITE OGWARI 11 3 OGARI PRY SCH.OGWARI OGARI PRY SCH.OGWARI 4 IGBAKWU 4 ISIOKWE ARA,IGBAKWU ISIOKWE ARA,IGBAKWU 5 OMASI 5 CENT. -

World Bank Document
Public Disclosure Authorized RURAL ACCESS AND AGRICULTURAL MARKETING PROJECT (RAAMP) RESETTLEMENT POLICY FRAMEWORK Public Disclosure Authorized FINAL REPORT Public Disclosure Authorized Federal Project Management Unit (FPMU) Federal Ministry of Agriculture and Rural Development NAIC House, Plot 590, Zone AO, Airport Road, Central Area, Abuja Public Disclosure Authorized May, 2018 LIST OF ABBREVIATIONS AND ACRONYMS AFD French Development Agency ARAP Abbreviated Resettlement Action Plan FPMU Federal Project Management Unit GIS Geographical Information System GPS Global Positioning System LGA Local Government Area NEEDS National Economic Empowerment and Development Strategy NPIRD National Policy on Integrated Rural Development NPRTT National Policy on Rural Travel and Transport NTP National Transport Policy PAPs Project Affected Persons PMU Project Management Unit RAAMP Rural Access and Agricultural Marketing Project RAP Resettlement Action Plan RTTP Rural Travel and Transport Policy SEEDS State Economic Empowerment and Development Strategy SPIU State Project Implementation Unit WB World Bank 2 EXECUTIVE SUMMARY Introduction The Federal Government of Nigeria has initiated the preparation of the Rural Access and Agricultural Marketing Project (RAAMP) the successor of the Second Rural Access and Mobility Project (RAMP-2). The project will be supported with financing from the World Bank and French Development Agency (AFD) and will be guided by the Government’s Rural Travel and Transport Policy (RTTP). The lead agency for the Federal Government is the Federal Department of Rural Development (FDRD) of the Federal Ministry of Agriculture and Rural Development (FMARD). The Federal Project Management Unit (FPMU) is overseeing the project on behalf of the FDRD, while the respective selected States Government will be responsible for the implementation of the project. -

Information Needs of Rural Dwellers As a Measure of the Effectiveness of Library and Information Services Provision in Anambra State, Nigeria Chinwe V
Information Needs of Rural Dwellers as a Measure of the Effectiveness of Library and Information Services Provision in Anambra State, Nigeria Chinwe V. Anunobi- PhD1, A. U. Ogbonna2, & N. P. Osuchukwu3 Nnamdi Azikiwe University Awka , Nigeria Justice Development and Peace Library, Onitsha [email protected], [email protected], & [email protected] Abstract Purpose: The study was designed to determine the effectiveness of information services provision to rural dwellers in Anambra State, Nigeria vis-a-vis their information needs. Design/Methodology: A survey was carried out using rural dwellers in Anambra North Senatorial Zone of Anambra State including civil servants, petty traders, farmers, artisans( welders, carpenters, hairdressers, carpenters) and the unemployed. The survey employed three instruments to elicit data from library and information centres (LIC) and the rural dwellers in Anambra North Senatorial Zone of Anambra State. Questionnaire and observation checklist were designed for data collection from the LIC. Questionnaire was provided to the managers of available LIC in the Zone namely: Nteje Community Development Information Centre, Igbariam Community Development Information Centre, Awkuzu Central Library and Atani Community Branch Library. Observation checklist was used for on- the- spot check of the available resources as well as data on the statistics of the users and resources consulted. The predominantly illiterate rural community in Nigeria justifies the use of interview to elicit information from rural dwellers on their awareness of information centres, their use, as well as approach to their information sourcing and needs. A total of eighty (80) respondents were interviewed from the communities, twenty (20) respondents per community. These rural dwellers were randomly sampled in the community. -

(ESMP) for the Amachalla Gully Erosion Site
Nigeria Erosion and Watershed Management Project (NEWMAP) Draft Report Public Disclosure Authorized Environmental and Social Management Plan (ESMP) for the Amachalla Gully Erosion Site Awka South, Anambra State. Public Disclosure Authorized Public Disclosure Authorized [Document title] [Document subtitle] Abstract [Draw your reader in with an engaging abstract. It is typically a short summary of the document. When you’re ready to add your content, just click here and start typing.] Public Disclosure Authorized Prepared for the State Project Management Unit (SPMU) Anambra State Nigeria Erosion and Watershed Management Project [Email address] Draft Report for the Amachalla gully erosion site Nigeria Erosion and Watershed Management Project (NEWMAP) Draft Report Environmental and Social Management (ESMP) for the Amachalla Gully Erosion Site Awka, Anambra State Prepared for the State Project Management Unit (SPMU) Anambra State Nigeria Erosion and Watershed Management Project ii Draft Report for the Amachalla gully erosion site Table of Contents LIST OF FIGURES ..................................................................................................................................... IV LIST OF PHOTOS ...................................................................................................................................... V LIST OF TABLES ....................................................................................................................................... V EXECUTIVE SUMMARY .............................................................................................................. -

EMERGENT MASCULINITIES: the GENDERED STRUGGLE for POWER in SOUTHEASTERN NIGERIA, 1850-1920 by Leonard Ndubueze Mbah a DISSERTAT
EMERGENT MASCULINITIES: THE GENDERED STRUGGLE FOR POWER IN SOUTHEASTERN NIGERIA, 1850-1920 By Leonard Ndubueze Mbah A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of History – Doctor of Philosophy 2013 ABSTRACT EMERGENT MASCULINITIES: THE GENDERED STRUGGLE FOR POWER IN SOUTHEASTERN NIGERIA, 1850-1920 By Leonard Ndubueze Mbah This dissertation uses oral history, written sources, and emic interpretations of material culture and rituals to explore the impact of changes in gender constructions on the historical processes of socio-political transformation among the Ohafia-Igbo of southeastern Nigeria between 1850 and 1920. Centering Ohafia-Igbo men and women as innovative historical actors, this dissertation examines the gendered impact of Ohafia-Igbo engagements with the Atlantic and domestic slave trade, legitimate commerce, British colonialism, Scottish Christian missionary evangelism, and Western education in the 19th and 20th centuries. It argues that the struggles for social mobility, economic and political power between and among men and women shaped dynamic constructions of gender identities in this West African society, and defined changes in lineage ideologies, and the borrowing and adaptation of new political institutions. It concludes that competitive performances of masculinity and political power by Ohafia men and women underlines the dramatic shift from a pre-colonial period characterized by female bread- winners and more powerful and effective female socio-political institutions, to a colonial period of male socio-political domination in southeastern Nigeria. DEDICATION To the memory of my father, late Chief Ndubueze C. Mbah, my mother, Mrs. Janet Mbah, my teachers and Ohafia-Igbo men and women, whose forbearance made this study a reality. -

ORAL PERFORMANCE AS SIREN the Example of Ozoemena
ORAL PERFORMANCE AS SIREN Alex Asigbo, PhD The Example of Ozoemena Nwa Nsugbe time. For the people of Omambla1 (old Anambra L.G.A.) various types of music exist. By the nature of Egwu Ekpili music, it is best Alex Asigbo, PhD suited for purposes of extolling virtues and condemning deviance. Theatre Arts Department Nnamdi Azikiwe University This is because, the light instruments allow for maneuverability Awka, Nigeria. hence the musicians can follow whoever is their butt about. It is a form best suited for wandering minstrels in that it requires sparse instruments to complete the ensemble. A typical Egwu Ekpili requires Ekpili (Rattles), Igba (drum), Udu (water pot) and Abstract sometimes Ogidi (long wooden drum) and Ekwe (gong). The Marxist literature pioneered literature of commitment or what practitioners of Egwu Ekpili today are the last of the wandering the French call “Literattu Engage”. Committed literature or art, minstrels. presupposes that a work of art must be devoted to the espousal of It is this nature of Egwu Ekpili that makes it best suited for the a specific ideology; preferably that which furthers the cause of the propagation of new ideas and or condemning of deviant behavior. down-trodden. Marxism influenced and is still influencing the Fully aware of the potentials of his music, Ozoemena was able to works of the second generation Nigerian playwrights or those turn his art into a mercantile venture by pandering to the wishes whose works gained ascendancy in the 1970s. During the 1970s of wealthy clients through placing his wit at their disposal. In also, Afro beat maestro, Fela Anikulapo- Kuti was using his music defending his penchant for praise singing, Ozoemena insists that not only to lampoon the ruling oligarchy but also to raise the people’s a hungry stomach brooks no morals hence he must first fill his consciousness. -

Global Journal of Geological Science (Issn 1596 – 6798)
PALEODEPOSITIONAL ENVIRONMENTAL DIAGNOSIS OF THE NSUGBE AREA AND ENVIRONS, ANAMBRA STATE, SOUTHEASTERN NIGERIA: AN APPLICATION OF PEBBLE MORPHOMETRIC STUDIES. B. N. NFOR (Received 17 April, 2009; Revision Accepted 2 September, 2009) ABSTRACT The application of pebble morphometric studies in diagnosing the paleodepositional environment of Nsugbe area and environs was carried out using one hundred and seventy (170) vein quartz recovered from seventeen (17) outcrops widely distributed in the studied area. Results show that the pebbles are rounded to subrounded and dominantly compact to compact-bladed. Computed mean values for the flatness index (FI), elongation, maximum projection sphericity index (MPSI) and oblate-prolate index (OPI) are 0.47, 0.74, 0.67 and 0.40 respectively. When these values were integrated with bivariate plots of MPSI versus OPI and mean roundness versus elongation ratio, a dominantly fluviatile depositional environment with subordinate beach/littoral setting are indicated for the Nsugbe area and environs. KEY WORDS: Pebble morphometry, Paleodepositional environment, Fluviatile, Nsugbe, Anambra basin. INTRODUCTION in Southern Nigeria though they are lateral equivalents. Nfor (2008) has however, frowned at the continuous Paleodepositional environments have been usage and designation of the terminology ‘Nsugbe determined using grain size analyses. The basic Formation’ in defiance of the due procedures prescribed philosophy of this technique is that the sediment type by the International Commission on Stratigraphic (continental, intermediate and marine), can be Nomenclature (ICSN). distinguished from each other by careful statistical evaluation of their texture; Folk (1967), Moiola and Location and Brief Geology of the Study Area Weiser (1968), Petitjohn et al. (1972), Freidman (1961, The study area covers a surface area of 55km2 1967, 1979) and Nwajide and Hoque (1982). -

Spatial Statistics of Poultry Production in Anambra State of Nigeria: a Preliminary for Bio-Energy Plant Location Modelling
Nigerian Journal of Technology (NIJOTECH) Vol. 35, No. 4, October 2016, pp. 940 – 948 Copyright© Faculty of Engineering, University of Nigeria, Nsukka, Print ISSN: 0331-8443, Electronic ISSN: 2467-8821 www.nijotech.com http://dx.doi.org/10.4314/njt.v35i4.32 SPATIAL STATISTICS OF POULTRY PRODUCTION IN ANAMBRA STATE OF NIGERIA: A PRELIMINARY FOR BIO-ENERGY PLANT LOCATION MODELLING E. C. Chukwuma1,*, L. C. Orakwe2, D. C. Anizoba3 and A. I. Manumehe4 1,2,3 DEPT. OF AGRIC. & BIORESOURCES ENGINEERING NNAMDI AZIKIWE UNIVERSITY, AWKA, ANAMBRA STATE, NIGERIA 4 NATIONAL ENVIRONMENTAL STANDARDS & REGULATIONS ENFORCEMENT AGENCY, AWKA, ANAMBRA STATE, NIGERIA E-mail addresses: 1 [email protected], 2 [email protected], 3 [email protected], 4 [email protected] ABSTRACT Consequent on the need to utilize bio-wastes for energy generation, a preliminary study for bio-energy plant location modelling was carried out in this work using spatial statistics technique in Anambra State of Nigeria as a case study. Spatial statistics toolbox in ArcGIS was used to generate point density map which reveal the regional patterns of biomass distribution and to generate hotspot analysis of zones of poultry production sites in the study area. The result of the study indicates that the central regions of the state is characterised with high point density poultry production sites, some of the locations with high point density poultry production values include: Ogbaru town which has the highest point density value of above 4,362,480kg of poultry droppings. This is followed by Umuchu, Onitsha, Nise, Nibo and Amawbia, with point density value that ranges from 2,210,250kg to 4,362,480kg. -

Priest Personnel Placement
PERSONNEL PLACEMENT 2020/2021 CATHOLIC ARCHDIOCESE OF ONITSHA, NIGERIA. Archdiocesan Catholic Secretariat, PO Box 411, Onitsha, Anambra State, Nigeria E-mail: [email protected] Website: http://www.onitsha-archdiocese.org ARCHBISHOP’S OFFICE SCHEDULE. For the Lay Faithful (Every Tuesday; 9a.m.-1p.m) For Priests and Religious Men and Women (Every Wednesday, 9a.m.-1p.m) Note: Other days are strictly on appointment. The Secretaries to the Archbishop are on seat for business on these other days. ARCHBISHOP: His Excellency, Archbishop Valerian M. OKEKE. AUXILIARY BISHOP His Excellency, Bishop Denis C. ISIZOH AUXILIARY BISHOP’S OFFICE SCHEDULE. At Onitsha Catholic Secretariat Office (Every Wednesday; 9a.m.-1p.m). At St. Joseph’s Aguleri Office (Every Monday/Tuesday, 9a.m.-2p.m) Note: Other days are strictly on appointment. The Secretaries to the auxiliary bishop are on seat for business on these other days. ARCHDIOCESAN CURIA: GENERAL DUTIES: 01. Vicar General: Most Rev. Denis Chidi ISIZOH 02. Chancellor & Secretary to the Archdiocese: Fr. Prudentius AROH 03. Vice-Chancellor & Coordinator, Health Services: Fr. Basil EKWUNIFE. 04. College of Consultors: (i) Archbishop Valerian M. OKEKE (ii)Vicar General: Bishop Denis Chidi ISIZOH (iii) Chancellor: Fr. Prudentius AROH (iv) Vice Chancellor: Fr. Basil EKWUNIFE (v) All the Six Episcopal Vicars (vi) Rector of the Basilica 05. Presbyteral Council Members: Archbishop Valerian M. OKEKE Bishop Denis Chidi ISIZOH Msgr. Basil ONWUASOMBA Fr. Prudentius AROH Fr. Basil EKWUNIFE Fr. Patrick OMUTAH Fr. Matthew OBIEKEZIE Fr. Emmanuel EGWUOBA Fr. John Bosco EZIKA Fr. Aloysius IKEKWE Fr. Vincent ONUCHUKWU Fr. Nicholas A. AKWANYA Fr. Emmanuel NDUBISI, BSS.