Second Quarter 2019 Update: Changes to the Highmark Drug Formularies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novel Neuroprotective Compunds for Use in Parkinson's Disease
Novel neuroprotective compounds for use in Parkinson’s disease A thesis submitted to Kent State University in partial Fulfillment of the requirements for the Degree of Master of Science By Ahmed Shubbar December, 2013 Thesis written by Ahmed Shubbar B.S., University of Kufa, 2009 M.S., Kent State University, 2013 Approved by ______________________Werner Geldenhuys ____, Chair, Master’s Thesis Committee __________________________,Altaf Darvesh Member, Master’s Thesis Committee __________________________,Richard Carroll Member, Master’s Thesis Committee ___Eric_______________________ Mintz , Director, School of Biomedical Sciences ___Janis_______________________ Crowther , Dean, College of Arts and Sciences ii Table of Contents List of figures…………………………………………………………………………………..v List of tables……………………………………………………………………………………vi Acknowledgments.…………………………………………………………………………….vii Chapter 1: Introduction ..................................................................................... 1 1.1 Parkinson’s disease .............................................................................................. 1 1.2 Monoamine Oxidases ........................................................................................... 3 1.3 Monoamine Oxidase-B structure ........................................................................... 8 1.4 Structural differences between MAO-B and MAO-A .............................................13 1.5 Mechanism of oxidative deamination catalyzed by Monoamine Oxidases ............15 1 .6 Neuroprotective effects -

(12) Patent Application Publication (10) Pub. No.: US 2013/0253056A1 Nemas Et Al
US 20130253 056A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0253056A1 Nemas et al. (43) Pub. Date: Sep. 26, 2013 (54) CONTINUOUS ADMINISTRATION OF (60) Provisional application No. 61/179,511, filed on May LEVODOPA AND/OR DOPA 19, 2009. DECARBOXYLASE INHIBITORS AND COMPOSITIONS FOR SAME Publication Classification (71) Applicant: NEURODERM, LTD., Ness-Ziona (IL) (51) Int. Cl. A63L/216 (2006.01) (72) Inventors: Mara Nemas, Gedera (IL); Oron (52) U.S. Cl. Yacoby-Zeevi, Moshav Bitsaron (IL) CPC .................................... A6 IK3I/216 (2013.01) USPC .......................................................... 514/538 (73) Assignee: Neuroderm, Ltd., Ness-Ziona (IL) (57) ABSTRACT (21) Appl. No.: 13/796,232 Disclosed herein are for example, liquid aqueous composi (22) Filed: Mar 12, 2013 tions that include for example an ester or salt of levodopa, or an ester or salt of carbidopa, and methods for treating neuro Related U.S. Application Data logical or movement diseases or disorders such as restless leg (63) Continuation-in-part of application No. 12/961,534, syndrome, Parkinson's disease, secondary parkinsonism, filed on Dec. 7, 2010, which is a continuation of appli Huntington's disease, Parkinson's like syndrome, PSP. MSA, cation No. 12/836,130, filed on Jul. 14, 2010, now Pat. ALS, Shy-Drager syndrome, dystonia, and conditions result No. 7,863.336, which is a continuation of application ing from brain injury including carbon monoxide or manga No. 12/781,357, filed on May 17, 2010, now Pat. No. nese intoxication, using Substantially continuous administra 8,193,243. tion of levodopa and/or carbidopa or ester and/or salt thereof. -

PSP: Some Answers
PSP: Some Answers Lawrence I. Golbe, MD Professor of Neurology, Rutgers Robert Wood Johnson Medical School Director of Clinical Affairs and Scientific Advisory Board Chairman, CurePSP October 2017 What is Progressive Supranuclear Palsy (PSP)? Of the approximately five to seven of every 100,000 people in Canada with progressive supranuclear palsy (PSP), few, if any, had ever heard of the disease before their diagnosis. In fact, most patients with PSP report that their family doctors knew nothing about it until a neurologist made the diagnosis. As of now, three of every four people with a diagnosis of PSP could have been diagnosed earlier, if their doctor had suspected it and performed the appropriate examination. However, it is appearing in medical journals more and more often, which will help doctors become familiar with PSP. This pamphlet should help patients and their families do the same. Why has no one heard of PSP? PSP is rare: no one even realized it existed until 1963, when several patients were first described at a national neurology research convention and the disease was given its name. In retrospect, at least 12 cases of PSP had appeared in the medical literature between 1909 and 1962, but because of its resemblance to Parkinson’s, it wasn’t recognized as a distinct disease. The brain under the microscope is almost identical to that of “post-encephalitic parkinsonism,” a common condition in the early 20th century but now nearly extinct, which also made for erroneous diagnoses during that era. Although PSP is slightly more common than the well-known amyotrophic lateral sclerosis (called ALS, or Lou Gehrig’s disease in the U.S. -

Summary of Drug Limitations Mary C
RON DESANTIS GOVERNOR SUMMARY OF DRUG LIMITATIONS MARY C. MAYHEW SECRETARY **Medications listed in this document may or may not require a prior authorization. Please view the Preferred Drug List at: http://ahca.myflorida.com/Medicaid/Prescribed_Drug/pharm_thera/fmpdl.shtml** Summary of Drug Limitations Abilify (aripiprazole) 2mg, 5mg, 20mg, 30mg tablets Minimum age = 6; Maximum of 1 tablet per day Abilify (aripiprazole) 10mg, 15mg tablets Minimum age = 6; Maximum of 15mg per day for ages = 6 - 11; Maximum of 30mg per day for ages = 12-17 Maximum of 1 tablet per day Abilify (aripiprazole) Discmelt 10mg, 15mg tabs Minimum age = 6; Maximum of 15mg per day for ages = 6 - 11; Maximum of 30mg per day for ages = 12-17; Maximum of 2 tablets per day Abilify (aripiprazole) 1mg/ml solution Minimum age = 6; Maximum of 15ml per day for ages = 6 - 11; Maximum of 30ml per day for ages = 12-17; Maximum of 30ml per day for ages =/> 18 Abilify Maintena (aripiprazole) syringe/vial Minimum age = 18; Maximum of 1 syringe or vial every 28 days Absorica (isotretinoin) 10mg, 20mg, 25mg,30mg, 35mg, & Minimum age = 12 40mg capsules Abstral (fentanyl citrate) sublingual tablets Minimum age = 18; Maximum of 4 sublingual tablets per day Acanya (benzoyl peroxide/clindamycin)Gel, gel pump Minimum Age= 12 Accolate (zafirlukast) tablets Maximum of 3 tablets per day Aciphex (rabeprazole) 5mg, 10mg sprinkle capsules Minimum age = 1; Maximum age = 11; Maximum of 1 capsule per day Aciphex (rabeprazole) 20mg tablets Minimum age = 1; Maximum of 2 tablets per day Actemra (tocilizumab) 80mg/4ml, 200mg/10ml, Minimum age= 2 400mg/20ml Vials, & 162mg/0.9ml Syringe Actimmune (Interferon Gamma-1b) Maximum of 6ml every 28days Actiq (fentanyl citrate) Lozenges Minimum age = 18; Maximum of 4 lozenges per day Activella (estradiol/norethindrone) tablets Minimum age = 18 Updated 02/28/2019 1 RON DESANTIS GOVERNOR SUMMARY OF DRUG LIMITATIONS MARY C. -

XERMELO™ (Telotristat Ethyl)
PHARMACY COVERAGE GUIDELINES ORIGINAL EFFECTIVE DATE: 5/18/2017 SECTION: DRUGS LAST REVIEW DATE: 5/20/2021 LAST CRITERIA REVISION DATE: 5/20/2021 ARCHIVE DATE: XERMELO™ (telotristat ethyl) Coverage for services, procedures, medical devices and drugs are dependent upon benefit eligibility as outlined in the member's specific benefit plan. This Pharmacy Coverage Guideline must be read in its entirety to determine coverage eligibility, if any. This Pharmacy Coverage Guideline provides information related to coverage determinations only and does not imply that a service or treatment is clinically appropriate or inappropriate. The provider and the member are responsible for all decisions regarding the appropriateness of care. Providers should provide BCBSAZ complete medical rationale when requesting any exceptions to these guidelines. The section identified as “Description” defines or describes a service, procedure, medical device or drug and is in no way intended as a statement of medical necessity and/or coverage. The section identified as “Criteria” defines criteria to determine whether a service, procedure, medical device or drug is considered medically necessary or experimental or investigational. State or federal mandates, e.g., FEP program, may dictate that any drug, device or biological product approved by the U.S. Food and Drug Administration (FDA) may not be considered experimental or investigational and thus the drug, device or biological product may be assessed only on the basis of medical necessity. Pharmacy Coverage Guidelines are subject to change as new information becomes available. For purposes of this Pharmacy Coverage Guideline, the terms "experimental" and "investigational" are considered to be interchangeable. BLUE CROSS®, BLUE SHIELD® and the Cross and Shield Symbols are registered service marks of the Blue Cross and Blue Shield Association, an association of independent Blue Cross and Blue Shield Plans. -

Azilect, INN-Rasagiline
SCIENTIFIC DISCUSSION 1. Introduction AZILECT is indicated for the treatment of idiopathic Parkinson’s disease (PD) as monotherapy (without levodopa) or as adjunct therapy (with levodopa) in patients with end of dose fluctuations. Rasagiline is administered orally, at a dose of 1 mg once daily with or without levodopa. Parkinson’s disease is a common neurodegenerative disorder typified by loss of dopaminergic neurones from the basal ganglia, and by a characteristic clinical syndrome with cardinal physical signs of resting tremor, bradikinesia and rigidity. The main treatment aims at alleviating symptoms through a balance of anti-cholinergic and dopaminergic drugs. Parkinson’s disease (PD) treatment is complex due to the progressive nature of the disease, and the array of motor and non-motor features combined with early and late side effects associated with therapeutic interventions. Rasagiline is a chemical inhibitor of the enzyme monoamine oxidase (MAO) type B which has a major role in the inactivation of biogenic and diet-derived amines in the central nervous system. MAO has two isozymes (types A and B) and type B is responsible for metabolising dopamine in the central nervous system; as dopamine deficiency is the main contributing factor to the clinical manifestations of Parkinson’s disease, inhibition of MAO-B should tend to restore dopamine levels towards normal values and this improve the condition. Rasagiline was developed for the symptomatic treatment of Parkinson’s disease both as monotherapy in early disease and as adjunct therapy to levodopa + aminoacids decarboxylase inhibitor (LD + ADI) in patients with motor fluctuations. 2. Quality Introduction Drug Substance • Composition AZILECT contains rasagiline mesylate as the active substance. -

Compositions for Continuous Administration of Dopa
(19) TZZ ¥ _T (11) EP 2 432 454 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/08 (2006.01) A61K 9/10 (2006.01) 01.03.2017 Bulletin 2017/09 A61K 31/198 (2006.01) A61P 25/16 (2006.01) (21) Application number: 10725880.8 (86) International application number: PCT/IL2010/000400 (22) Date of filing: 17.05.2010 (87) International publication number: WO 2010/134074 (25.11.2010 Gazette 2010/47) (54) COMPOSITIONS FOR CONTINUOUS ADMINISTRATION OF DOPA DECARBOXYLASE INHIBITORS ZUSAMMENSETZUNGEN FÜR DIE KONTINUIERLICHE VERABREICHUNG VON DOPA-DECARBOXYLASEHEMMERN COMPOSITIONS POUR ADMINISTRATION CONTINUE D’UN INHIBITEUR DE LA DOPA-DÉCARBOXYLASE (84) Designated Contracting States: (74) Representative: ABG Patentes, S.L. et al AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Avenida de Burgos, 16D GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Edificio Euromor PL PT RO SE SI SK SM TR 28036 Madrid (ES) (30) Priority: 19.05.2009 US 179511 P (56) References cited: WO-A1-2006/006929 US-A- 4 409 233 (43) Date of publication of application: 28.03.2012 Bulletin 2012/13 • NYHOLM D: "Enteral levodopa/carbidopa gel infusion for the treatment of motor fluctuations (73) Proprietor: Neuroderm Ltd and dyskinesias in advanced Parkinson’s 74036 Ness Ziona (IL) disease" EXPERT REVIEW OF NEUROTHERAPEUTICS, FUTURE DRUGS, (72) Inventors: LONDON, GB LNKD- • YACOBY-ZEEVI, Oron DOI:10.1586/14737175.6.10.1403, vol. 6, no. 10, 1 60946 Bitsaron (IL) January 2006 (2006-01-01), pages 1403-1411, •NEMAS,Mara XP008082627 ISSN: 1473-7175 70700 Gedera (IL) Remarks: Thefile contains technical information submitted after the application was filed and not included in this specification Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

Oral Systemic Therapy Pharmacy Toolkit
Oral Systemic Therapy Pharmacy Toolkit PROCARBAZINE INSTRUCTIONS FOR THE PHARMACIST Prescription • All orders should be written on a pre-printed order; if not, compare prescription to standard regimens in the Systemic Therapy Manual to confirm the dosing and instructions o The order must be signed by BOTH the prescriber (at the bottom) AND at least one other oncology health professional (nurse or hospital pharmacist) who has verified the order o Measure the patient’s height (cm) and weight (Kg), then recalculate body surface area (BSA) • The prescription may not be refilled (unless specifically ordered by the oncologist) and it may not be filled as a continuing care prescription o If the prescriber has written for refills, do not dispense until the oncology team authorizes the refill; Blood work must be checked for each cycle. • Always check for drug-drug interactions, especially before the first cycle, as described below. Consult the Drug Interactions section (page 4), and consider an online drug interactions checking program. • Check with patient for any other medications filled at a different pharmacy Handling and Dispensing • When handling this drug, disposable gloves should be worn at all times by any woman of child-bearing potential. Counting trays and other equipment directly exposed to the drug should be cleaned with a sodium hypochlorite (bleach) solution (or soap and water), followed by rinsing with copious amounts of water (wear gloves). Do not open capsules in an open air environment and risk inhalation of powder. • ALWAYS affix the auxiliary label to identify this medication as “Cancer Chemotherapy”- this is an important warning label for other health professionals caring for the patient. -

New Drugs Approved in FY 2014
New Drugs Approved in FY 2014 New Active Ingredient(s) Review Brand Name Approval/ Approval Date No. (underlined: new active Notes Category (Applicant Company) Partial ingredient) Change 1 Jul. 4, 2014 1 Dovobet Ointment Approval (1) Calcipotriol A new combination drug indicated for the treatment (Leo Pharma K.K.) hydrate/ of psoriasis vulgaris. (2) Betamethasone dipropionate 1 Aug. 29, 2014 2 Rituxan Injection 10 mg/mL Change Rituximab (genetical A drug with a new additional indication and a new (Zenyaku Kogyo Co., Ltd.) recombination) dosage for the treatment of refractory nephrotic syndrome (for use in patients with frequent recurrence or steroid-dependent). [Orphan drug] 1 Sep. 19, 2014 3 Thymoglobuline for Intravenous Infusion 25 mg Change Anti-human thymocyte A drug with a new additional indication and a new (Sanofi K.K.) immunoglobulin, rabbit dosage for the treatment of acute rejection after the liver, heart, lungs, pancreas, or small intestinal transplantation. 1 Sep. 26, 2014 4 Fomepizole Intravenous Infusion 1.5 g "Takeda" Approval Fomepizole A drug with a new active ingredient indicated for the (Takeda Pharmaceutical Company Limited) treatment of ethylene glycol and methanol poisonings. 1 Dec. 26, 2014 5 Pariet Tablets 5 mg Approval Rabeprazole sodium A drug with a new additional indication and a new Pariet Tablets 10 mg Change dosage in a newly-added dosage form, and a drug (Eisai Co., Ltd.) with a new additional indication and a new dosage for the prevention of recurrence of gastric ulcer or duodenal ulcer in patients treated -

Opicapone for the Management of End-Of-Dose Motor Fluctuations in Patients with Parkinson’S Disease Treated with L-DOPA
Opicapone for the management of end-of-dose motor fluctuations in patients with Parkinson’s disease treated with L-DOPA Andrew J. Lees MD1, Joaquim Ferreira MD2, Olivier Rascol MD3, Heinz Reichmann MD,4 Fabrizio Stocchi MD,5 Eduardo Tolosa MD,6 Werner Poewe MD7 1. University College London, Reta Lila Weston Institute, London, UK 2. Hospital de Santa Maria, Centro de Estudos Egas Moniz, Lisbon, Portugal 3. Departments of Clinical Pharmacology and Neurosciences, Clinical Investigation Center CIC 1436, NS-Park/FCRIN network and NeuroToul COEN Center, INSERM, Toulouse University Hospital and Toulouse3 University, Toulouse, France 4. Department of Neurology, Technische Universitaet Dresden, Dresden, Germany 5. Institute of Neurology, IRCCS San Raffaele Pisana, Rome, Italy 6. Neurology Service, Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED), Hospital Clínic, IDIBAPS, Universitat de Barcelona, Spain. 7. Department of Neurology, Innsbruck Medical University, Innsbruck, Austria Corresponding author Professor Andrew Lees University College London, Reta Lila Weston Institute, 1 Wakefield Street London WC1N 1PJ London, UK Email: [email protected] Direct telephone: + 44 20 7xxxxxxx Fax: +44 20 7xxxxxxx 1 Joaquim Ferreira [email protected] Olivier Rascol [email protected] Eduardo Tolosa [email protected] Fabrizio Stocchi [email protected] Heinz Reichmann [email protected] Werner Poewe [email protected] 2 Summary Introduction: Opicapone is a third generation, highly potent and effective catechol O‑methyltransferase (COMT) inhibitor that optimizes the pharmacokinetics and bioavailability of L- DOPA therapy. Areas covered: In this review, we describe the preclinical and clinical development of opicapone. -
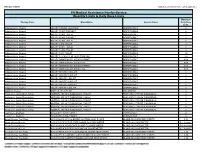
Quantity Limits/Daily Dose Limits
Effective 07/20/21 Alphabetical by Brand Name (when applicable) PA Medical Assistance Fee-for-Service Quantity Limits & Daily Dose Limits Maximum Therapy Class Brand Name Generic Name Daily Dose Limit Antipsychotics, Atypical ABILIFY 1 MG/ML SOLUTION ARIPIPRAZOLE 25 Antipsychotics, Atypical ABILIFY 10 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 10 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 15 MG DISCMELT ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 15 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 2 MG TABLET ARIPIPRAZOLE 2 Antipsychotics, Atypical ABILIFY 20 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 30 MG TABLET ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY 5 MG TABLET ARIPIPRAZOLE 1.5 Antipsychotics, Atypical ABILIFY 9.75 MG/1.3 ML INJECTION VIAL ARIPIPRAZOLE 3.9 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 300 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG SYRINGE ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MAINTENA ER 400 MG VIAL ARIPIPRAZOLE 0.04 Antipsychotics, Atypical ABILIFY MYCITE 10 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 15 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 2 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 20 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 30 MG KIT ARIPIPRAZOLE 1 Antipsychotics, Atypical ABILIFY MYCITE 5 MG KIT ARIPIPRAZOLE 1 Antivirals, Herpes ABREVA 10% -

207145Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 207145Orig1s000 MEDICAL REVIEW(S) Clinical Review Leonard P. Kapcala, M.D. NDA 207145 XADAGO (SAFINAMIDE) CLINICAL REVIEW Application Type NDA Application Number 207145 Priority or Standard Standard Submit Date 9/21/16 Received Date 9/21/16 PDUFA Goal Date 3/21/17 Division / Office DNP/ODE 1 Reviewer Name Leonard P. Kapcala, M.D. Review Completion Date 2/7/17 Established Name SAFINAMIDE (Proposed) Trade Name XADAGO Therapeutic Class Monoamine Oxidase B Inhibitor Applicant Newron Formulation(s) Tablet Dosing Regimen Once daily orally Indication(s) Treatment of signs and symptoms of Parkinson's disease Intended Population(s) Patients with early and advanced Parkinson's disease 1 Reference ID: 4071416 Clinical Review Leonard P. Kapcala, M.D. NDA 207145 XADAGO (SAFINAMIDE) Table of Contents 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT ......................................... 4 1.1 Recommendation on Regulatory Action ............................................................. 4 1.2 Risk Benefit Assessment .................................................................................... 4 1.3 Recommendations for Postmarket Risk Evaluation and Mitigation Strategies ... 5 1.4 Recommendations for Postmarket Requirements and Commitments ................ 5 2 INTRODUCTION AND REGULATORY BACKGROUND ........................................ 5 2.1 Product Information (Based Upon Sponsor Summary) ....................................... 5 2.2 Other Relevant Background Information ...........................................................